Ex vivo gene transfer of viral interleukin-10 to BB rat islets: no protection after transplantation to diabetic BB rats
- PMID: 17760846
- PMCID: PMC3823263
- DOI: 10.1111/j.1582-4934.2007.00059.x
Ex vivo gene transfer of viral interleukin-10 to BB rat islets: no protection after transplantation to diabetic BB rats
Abstract
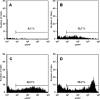
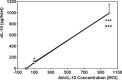
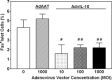
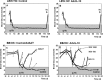
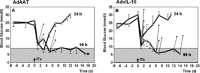
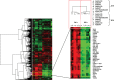
Allogeneic and autoimmune islet destruction limits the success of islet transplantation in autoimmune diabetic patients. This study was designed to investigate whether ex vivo gene transfer of viral interleukin-10 (vIL-10) protects BioBreeding (BB) rat islets from autoimmune destruction after transplantation into diabetic BB recipients. Islets were transduced with adenoviral constructs (Ad) expressing the enhanced green fluorescent protein (eGFP), alpha-1 antitrypsin (AAT) or vIL-10. Transduction efficiency was demonstrated by eGFP-positive cells and vIL-10 production. Islet function was determined in vitro by measuring insulin content and insulin secretion and in vivo by grafting AdvIL-10-transduced islets into syngeneic streptozotocin (SZ)-diabetic, congenic Lewis (LEW.1 W) rats. Finally, gene-modified BB rat islets were grafted into autoimmune diabetic BB rats. Ad-transduction efficiency of islets increased with virus titre and did not interfere with insulin content and insulin secretion. Ad-transduction did not induce Fas on islet cells. AdvIL-10-transduced LEW.1 W rat islets survived permanently in SZ-diabetic LEW.1 W rats. In diabetic BB rats AdvIL-10-transduced BB rat islets were rapidly destroyed. Prolongation of islet culture prior to transplantation improved the survival of gene-modified islets in BB rats. Several genes including those coding for chemokines and other peptides associated with inflammation were down-regulated in islets after prolonged culture, possibly contributing to improved islet graft function in vivo. Islets transduced ex vivo with vIL-10 are principally able to cure SZ-diabetic rats. Autoimmune islet destruction in diabetic BB rats is not prevented by ex vivo vIL-10 gene transfer to grafted islets. Graft survival in autoimmune diabetic rats may be enhanced by improvements in culture conditions prior to transplantation.
Figures
Similar articles
-
Viral IL-10 gene transfer prolongs rat islet allograft survival.Cell Transplant. 2008;17(6):609-18. doi: 10.3727/096368908786092694. Cell Transplant. 2008. PMID: 18819249
-
Interleukin-4 or interleukin-10 expressed from adenovirus-transduced syngeneic islet grafts fails to prevent beta cell destruction in diabetic NOD mice.Transplantation. 1997 Oct 15;64(7):1040-9. doi: 10.1097/00007890-199710150-00017. Transplantation. 1997. PMID: 9381527
-
Prevention of autoimmune islet allograft destruction by engraftment of donor T cells.Transplantation. 1997 Jan 27;63(2):299-303. doi: 10.1097/00007890-199701270-00021. Transplantation. 1997. PMID: 9020334
-
Initiation of autoimmune type 1 diabetes and molecular cloning of a gene encoding for islet cell-specific 37kd autoantigen.Adv Exp Med Biol. 1994;347:207-20. doi: 10.1007/978-1-4615-2427-4_18. Adv Exp Med Biol. 1994. PMID: 7976732 Review.
-
Ex vivo gene transfer for improvement of transplanted pancreatic islet viability and function.Curr Pharm Des. 2005;11(22):2927-40. doi: 10.2174/1381612054546743. Curr Pharm Des. 2005. PMID: 16101446 Review.
Cited by
-
Recombinant adenoviral expression of IL-10 protects beta cell from impairment induced by pro-inflammatory cytokine.Mol Cell Biochem. 2010 Nov;344(1-2):163-71. doi: 10.1007/s11010-010-0539-x. Epub 2010 Jul 25. Mol Cell Biochem. 2010. PMID: 20658311
-
Vaspin inhibits kallikrein 7 by serpin mechanism.Cell Mol Life Sci. 2013 Jul;70(14):2569-83. doi: 10.1007/s00018-013-1258-8. Epub 2013 Jan 31. Cell Mol Life Sci. 2013. PMID: 23370777 Free PMC article.
-
Effect of recombinant α1-antitrypsin Fc-fused (AAT-Fc)protein on the inhibition of inflammatory cytokine production and streptozotocin-induced diabetes.Mol Med. 2013 May 20;19(1):65-71. doi: 10.2119/molmed.2012.00308. Mol Med. 2013. PMID: 23552726 Free PMC article.
-
Pancreatic islet inflammation: an emerging role for chemokines.J Mol Endocrinol. 2017 Jul;59(1):R33-R46. doi: 10.1530/JME-17-0042. Epub 2017 Apr 18. J Mol Endocrinol. 2017. PMID: 28420714 Free PMC article. Review.
-
Multifunctional magnetic nanocarriers for image-tagged SiRNA delivery to intact pancreatic islets.Transplantation. 2008 Nov 15;86(9):1170-7. doi: 10.1097/TP.0b013e31818a81b2. Transplantation. 2008. PMID: 19005396 Free PMC article.
References
-
- Moore KW, De Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
-
- De Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, Te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, De Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes viadownregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. - PMC - PubMed
-
- Ralph P, Nakoinz I, Sampson-Johannes A, Fong S, Lowe D, Min HY, Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992;148:808–14. - PubMed
-
- Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–11. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

