Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty
- PMID: 17760861
- PMCID: PMC3363963
- DOI: 10.1111/j.1471-4159.2007.04882.x
Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty
Abstract
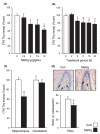
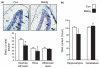
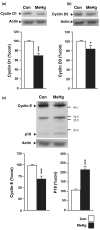
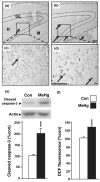
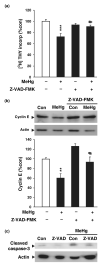
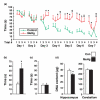
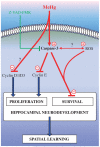
Normal brain development requires coordinated regulation of several processes including proliferation, differentiation, and cell death. Multiple factors from endogenous and exogenous sources interact to elicit positive as well as negative regulation of these processes. In particular, the perinatal rat brain is highly vulnerable to specific developmental insults that produce later cognitive abnormalities. We used this model to examine the developmental effects of an exogenous factor of great concern, methylmercury (MeHg). Seven-day-old rats received a single injection of MeHg (5 microg/gbw). MeHg inhibited DNA synthesis by 44% and reduced levels of cyclins D1, D3, and E at 24 h in the hippocampus, but not the cerebellum. Toxicity was associated acutely with caspase-dependent programmed cell death. MeHg exposure led to reductions in hippocampal size (21%) and cell numbers 2 weeks later, especially in the granule cell layer (16%) and hilus (50%) of the dentate gyrus defined stereologically, suggesting that neurons might be particularly vulnerable. Consistent with this, perinatal exposure led to profound deficits in juvenile hippocampal-dependent learning during training on a spatial navigation task. In aggregate, these studies indicate that exposure to one dose of MeHg during the perinatal period acutely induces apoptotic cell death, which results in later deficits in hippocampal structure and function.
Figures


Similar articles
-
Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E.Neurotoxicology. 2006 Dec;27(6):970-81. doi: 10.1016/j.neuro.2006.09.001. Epub 2006 Sep 15. Neurotoxicology. 2006. PMID: 17056119 Free PMC article.
-
N-acetyl cysteine treatment reduces mercury-induced neurotoxicity in the developing rat hippocampus.J Neurosci Res. 2012 Apr;90(4):743-50. doi: 10.1002/jnr.22819. J Neurosci Res. 2012. PMID: 22420031 Free PMC article.
-
Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits.Dev Neurobiol. 2013 Dec;73(12):936-49. doi: 10.1002/dneu.22119. Epub 2013 Sep 30. Dev Neurobiol. 2013. PMID: 23959606 Free PMC article.
-
MeHg Suppressed Neuronal Potency of Hippocampal NSCs Contributing to the Puberal Spatial Memory Deficits.Biol Trace Elem Res. 2016 Aug;172(2):424-436. doi: 10.1007/s12011-015-0609-8. Epub 2016 Jan 8. Biol Trace Elem Res. 2016. PMID: 26743863
-
Long-lasting neurotoxic effects of exposure to methylmercury during development.J Intern Med. 2013 May;273(5):490-7. doi: 10.1111/joim.12045. J Intern Med. 2013. PMID: 23600401 Review.
Cited by
-
Hippocampal developmental vulnerability to methylmercury extends into prepubescence.Front Neurosci. 2015 May 12;9:150. doi: 10.3389/fnins.2015.00150. eCollection 2015. Front Neurosci. 2015. PMID: 26029035 Free PMC article.
-
De novo synthesized estradiol protects against methylmercury-induced neurotoxicity in cultured rat hippocampal slices.PLoS One. 2013;8(2):e55559. doi: 10.1371/journal.pone.0055559. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405170 Free PMC article.
-
Early Developmental Low-Dose Methylmercury Exposure Alters Learning and Memory in Periadolescent but Not Young Adult Rats.Biomed Res Int. 2016;2016:6532108. doi: 10.1155/2016/6532108. Epub 2016 Jan 13. Biomed Res Int. 2016. PMID: 26885512 Free PMC article.
-
Animal models of autism spectrum disorders: information for neurotoxicologists.Neurotoxicology. 2009 Sep;30(5):811-21. doi: 10.1016/j.neuro.2009.07.002. Epub 2009 Jul 9. Neurotoxicology. 2009. PMID: 19596370 Free PMC article.
-
Prenatal arsenic exposure alters REST/NRSF and microRNA regulators of embryonic neural stem cell fate in a sex-dependent manner.Neurotoxicol Teratol. 2017 Jan-Feb;59:1-15. doi: 10.1016/j.ntt.2016.10.004. Epub 2016 Oct 14. Neurotoxicol Teratol. 2017. PMID: 27751817 Free PMC article.
References
-
- Alessandri B, Nishioka T, Heimann A, Bullock RM, Kempski O. Caspase-dependent cell death involved in brain damage after acute subdural hematoma in rats. Brain Res. 2006;1111:196–202. - PubMed
-
- Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J. Neurobiol. 2001;46:220–229. - PubMed
-
- Cheng Y, Black IB, DiCicco-Bloom E. Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur. J. Neurosci. 2002;15:3–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

