A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach
- PMID: 17760872
- PMCID: PMC2901540
- DOI: 10.1111/j.1471-4159.2007.04858.x
A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach
Abstract
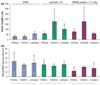
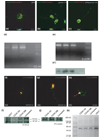
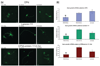
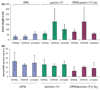
The failure of CNS regeneration and subsequent motor and sensory loss remain major unsolved questions despite massive accumulation of experimental observations and results. The sheer volume of data and the variety of resources from which these data are generated make it difficult to integrate prior work to build new hypotheses. To address these challenges we developed a prototypic suite of computer programs to extract protein names from relevant publications and databases and associated each of them with several general categories of biological functions in nerve regeneration. To illustrate the usefulness of our data mining approach, we utilized the program output to generate a hypothesis for a biological function of CD44 interaction with osteopontin (OPN) and laminin in axon outgrowth of CNS neurons. We identified CD44 expression in retinal ganglion cells and when these neurons were plated on poly-l-lysine 3% of them initiated axon growth, on OPN 15%, on laminin-111 (1x) 41%, on laminin-111 (0.5x) 56%, and on a mixture of OPN and laminin (1x) 67% of neurons generated axon growth. With the aid of a deoxyribozyme (DNA enzyme) to CD44 that digests the target mRNA, we demonstrated that a reduction of CD44 expression led to reduced axon initiation of retinal ganglion cells on all substrates. We suggest that such an integrative, applied systems biology approach to CNS trauma will be critical to understand and ultimately overcome the failure of CNS regeneration.
Figures


Similar articles
-
Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration.J Neurosci. 2012 May 23;32(21):7325-35. doi: 10.1523/JNEUROSCI.5472-11.2012. J Neurosci. 2012. PMID: 22623678 Free PMC article.
-
Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth.Brain. 2009 Nov;132(Pt 11):3102-21. doi: 10.1093/brain/awp240. Epub 2009 Sep 25. Brain. 2009. PMID: 19783665
-
Activated retinal glia mediated axon regeneration in experimental glaucoma.Neurobiol Dis. 2012 Jan;45(1):243-52. doi: 10.1016/j.nbd.2011.08.008. Epub 2011 Aug 10. Neurobiol Dis. 2012. PMID: 21867754
-
Return of function after CNS axon regeneration: Lessons from injury-responsive intrinsically photosensitive and alpha retinal ganglion cells.Prog Retin Eye Res. 2019 Jul;71:57-67. doi: 10.1016/j.preteyeres.2018.11.006. Epub 2018 Nov 17. Prog Retin Eye Res. 2019. PMID: 30458239 Review.
-
An integrin approach to axon regeneration.Eye (Lond). 2017 Feb;31(2):206-208. doi: 10.1038/eye.2016.293. Epub 2016 Dec 23. Eye (Lond). 2017. PMID: 28009347 Free PMC article. Review.
Cited by
-
RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: a resource for understanding the pathology at the systems level.PLoS One. 2013 Aug 9;8(8):e72567. doi: 10.1371/journal.pone.0072567. eCollection 2013. PLoS One. 2013. PMID: 23951329 Free PMC article.
-
The Retina of Osteopontin deficient Mice in Aging.Mol Neurobiol. 2018 Jan;55(1):213-221. doi: 10.1007/s12035-017-0734-9. Mol Neurobiol. 2018. PMID: 28866734 Free PMC article.
-
Inhibition of the plasma membrane Ca2+ pump by CD44 receptor activation of tyrosine kinases increases the action potential afterhyperpolarization in sensory neurons.J Neurosci. 2011 Feb 16;31(7):2361-70. doi: 10.1523/JNEUROSCI.5764-10.2011. J Neurosci. 2011. PMID: 21325503 Free PMC article.
-
Mouse mutants for the nicotinic acetylcholine receptor ß2 subunit display changes in cell adhesion and neurodegeneration response genes.PLoS One. 2011 Apr 25;6(4):e18626. doi: 10.1371/journal.pone.0018626. PLoS One. 2011. PMID: 21547082 Free PMC article.
-
Eye-hand laterality and right thoracic idiopathic scoliosis.Eur Spine J. 2014 Jun;23(6):1232-6. doi: 10.1007/s00586-014-3269-z. Epub 2014 Mar 17. Eur Spine J. 2014. PMID: 24633781
References
-
- Alfei L, Aita M, Caronti B, De Vita R, Margotto V, Medolago AL, Valente AM. Hyaluronate receptor CD44 is expressed by astrocytes in the adult chicken and in astrocyte cell precursors in early development of the chicken spinal cord. Eur. J. Histochem. 1999;43:29–38. - PubMed
-
- Alldinger S, Fonfara S, Kremmer E, Baumgartner W. Up-regulation of the hyaluronate receptor CD44 in canine distemper demyelinated plaques. Acta Neuropathol. 2000;99:138–146. - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

