Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes
- PMID: 17761517
- PMCID: PMC2413069
- DOI: 10.1152/ajpregu.00059.2007
Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes
Abstract
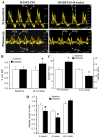
Isolated diastolic dysfunction is found in almost half of asymptomatic patients with well-controlled diabetes and may precede diastolic heart failure. However, mechanisms that underlie diastolic dysfunction during diabetes are not well understood. We tested the hypothesis that isolated diastolic dysfunction is associated with impaired myocardial Ca(2+) handling during type 1 diabetes. Streptozotocin-induced diabetic rats were compared with age-matched placebo-treated rats. Global left ventricular myocardial performance and systolic function were preserved in diabetic animals. Diabetes-induced diastolic dysfunction was evident on Doppler flow imaging, based on the altered patterns of mitral inflow and pulmonary venous flows. In isolated ventricular myocytes, diabetes resulted in significant prolongation of action potential duration compared with controls, with afterdepolarizations occurring in diabetic myocytes (P < 0.05). Sustained outward K(+) current and peak outward component of the inward rectifier were reduced in diabetic myocytes, while transient outward current was increased. There was no significant change in L-type Ca(2+) current; however, Ca(2+) transient amplitude was reduced and transient decay was prolonged by 38% in diabetic compared with control myocytes (P < 0.05). Sarcoplasmic reticulum Ca(2+) load (estimated by measuring the integral of caffeine-evoked Na(+)-Ca(2+) exchanger current and Ca(2+) transient amplitudes) was reduced by approximately 50% in diabetic myocytes (P < 0.05). In permeabilized myocytes, Ca(2+) spark amplitude and frequency were reduced by 34 and 20%, respectively, in diabetic compared with control myocytes (P < 0.05). Sarco(endo)plasmic reticulum Ca(2+)-ATPase-2a protein levels were decreased during diabetes. These data suggest that in vitro impairment of Ca(2+) reuptake during myocyte relaxation contributes to in vivo diastolic dysfunction, with preserved global systolic function, during diabetes.
Figures






References
-
- Abe T, Ohga Y, Tabayashi N, Kobayashi S, Sakata S, Misawa H, Tsuji T, Kohzuki H, Suga H, Taniguchi S, Takaki M. Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. Am J Physiol Heart Circ Physiol. 2002;282:H138–H148. - PubMed
-
- Akiyama N, Okumura K, Watanabe Y, Hashimoto H, Ito T, Ogawa K, Satake T. Altered acetylcholine and norepinephrine concentrations in diabetic rat hearts. Role of parasympathetic nervous system in diabetic cardiomyopathy. Diabetes. 1989;38:231–236. - PubMed
-
- Akula A, Kota MK, Gopisetty SG, Chitrapu RV, Kalagara M, Kalagara S, Veeravalli KK, Gomedhikam JP. Biochemical, histological and echocardiographic changes during experimental cardiomyopathy in STZ-induced diabetic rats. Pharmacol Res. 2003;48:429–435. - PubMed
-
- Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. - PubMed
-
- Belke DD, Dillmann WH. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep. 2004;6:424–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

