ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis
- PMID: 17761534
- PMCID: PMC2043549
- DOI: 10.1091/mbc.e07-03-0223
ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis
Abstract
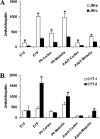
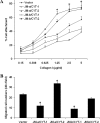
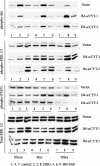
ErbB4, a member of the epidermal growth factor (EGF) receptor family that can be activated by heregulin beta1 and heparin binding (HB)-EGF, is expressed as alternatively spliced isoforms characterized by variant extracellular juxtamembrane (JM) and intracellular cytoplasmic (CYT) domains. ErbB4 plays a critical role in cardiac and neural development. We demonstrated that ErbB4 is expressed in the ureteric buds and developing tubules of embryonic rat kidney and in collecting ducts in adult. The predominant isoforms expressed in kidney are JM-a and CYT-2. In ErbB4-transfected MDCK II cells, basal cell proliferation and hepatocyte growth factor (HGF)-induced tubule formation were decreased by all four isoforms. Only JM-a/CYT-2 cells formed tubules upon HB-EGF stimulation. ErbB4 was activated by both HRG-beta1 and HB-EGF stimulation; however, compared with HRG-beta1, HB-EGF induced phosphorylation of the 80-kDa cytoplasmic cleavage fragment of the JM-a/CYT-2 isoform. HB-EGF also induced early activation of ERK1/2 in JM-a/CYT-2 cells and promoted nuclear translocation of the JM-a/CYT-2 cytoplasmic tail. In summary, our data indicate that JM-a/CYT-2, the ErbB4 isoform that is proteinase cleavable but does not contain a PI3K-binding domain in its cytoplasmic tail, mediates important functions in renal epithelial cells in response to HB-EGF.
Figures






Similar articles
-
Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth.Mol Biol Cell. 2006 Jan;17(1):67-79. doi: 10.1091/mbc.e05-05-0402. Epub 2005 Oct 26. Mol Biol Cell. 2006. PMID: 16251361 Free PMC article.
-
Role of membrane-bound heparin-binding epidermal growth factor-like growth factor (HB-EGF) in renal epithelial cell branching.Kidney Int. 2002 Jun;61(6):1968-79. doi: 10.1046/j.1523-1755.2002.00358.x. Kidney Int. 2002. PMID: 12028437
-
ErbB1 and ErbB4 generate opposing signals regulating mesenchymal cell proliferation during valvulogenesis.J Cell Sci. 2017 Apr 1;130(7):1321-1332. doi: 10.1242/jcs.196618. Epub 2017 Feb 23. J Cell Sci. 2017. PMID: 28232522
-
Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants.Trends Cardiovasc Med. 2000 Oct;10(7):304-10. doi: 10.1016/s1050-1738(01)00065-2. Trends Cardiovasc Med. 2000. PMID: 11343971 Review.
-
[Research progress of neuregulin 4 biological function].Sheng Li Xue Bao. 2017 Jun 25;69(3):351-356. Sheng Li Xue Bao. 2017. PMID: 28638929 Review. Chinese.
Cited by
-
Replication study for the association of 3 SNP loci identified in a genome-wide association study for diabetic nephropathy in European type 1 diabetes with diabetic nephropathy in Japanese patients with type 2 diabetes.Clin Exp Nephrol. 2013 Dec;17(6):866-71. doi: 10.1007/s10157-013-0797-5. Epub 2013 Mar 30. Clin Exp Nephrol. 2013. PMID: 23543049
-
Epidermal growth factor-mediated proliferation and sodium transport in normal and PKD epithelial cells.Biochim Biophys Acta. 2011 Oct;1812(10):1301-13. doi: 10.1016/j.bbadis.2010.10.004. Epub 2010 Oct 16. Biochim Biophys Acta. 2011. PMID: 20959142 Free PMC article. Review.
-
Nedd4 mediates ErbB4 JM-a/CYT-1 ICD ubiquitination and degradation in MDCK II cells.FASEB J. 2009 Jun;23(6):1935-45. doi: 10.1096/fj.08-121947. Epub 2009 Feb 4. FASEB J. 2009. PMID: 19193720 Free PMC article.
-
Parallel microarray profiling identifies ErbB4 as a determinant of cyst growth in ADPKD and a prognostic biomarker for disease progression.Am J Physiol Renal Physiol. 2017 Apr 1;312(4):F577-F588. doi: 10.1152/ajprenal.00607.2016. Epub 2017 Jan 11. Am J Physiol Renal Physiol. 2017. PMID: 28077374 Free PMC article.
-
The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology.Exp Cell Res. 2009 Feb 15;315(4):602-10. doi: 10.1016/j.yexcr.2008.08.005. Epub 2008 Aug 19. Exp Cell Res. 2009. PMID: 18761338 Free PMC article. Review.
References
-
- Balkovetz D. F. Hepatocyte growth factor and Madin-Darby canine kidney cells: in vitro models of epithelial cell movement and morphogenesis. Microsc. Res. Tech. 1998;43:456–463. - PubMed
-
- Beerli R. R., Hynes N. E. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J. Biol. Chem. 1996;271:6071–6076. - PubMed
-
- Boccaccio C., Ando M., Tamagnone L., Bardelli A., Michieli P., Battistini C., Comoglio P. M. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. - PubMed
-
- Brader S., Eccles S. A. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

