Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease
- PMID: 17761617
- PMCID: PMC2078678
- DOI: 10.1164/rccm.200702-334OC
Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease
Abstract
Rationale: Asthma is characterized by increases in airway resistance, pulmonary remodeling, and lung inflammation. The cytokine transforming growth factor (TGF)-beta has been shown to have a central role in asthma pathogenesis and in mouse models of allergic airway disease.
Objectives: To determine the contribution of TGF-beta to airway hyperresponsiveness (AHR), we examined the time course, source, and isoform specificity of TGF-beta production in an in vivo mouse asthma model. To then elucidate the function of TGF-beta in AHR, inflammation, and pulmonary fibrosis, we examined the effects of blocking TGF-beta signaling with neutralizing antibody.
Methods: Mice were sensitized and challenged with ovalbumin (OVA) to establish allergic airway disease. TGF-beta activity was neutralized by intranasal administration of monoclonal antibody.
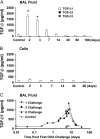
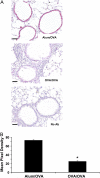
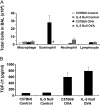
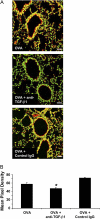
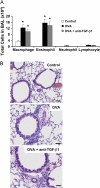
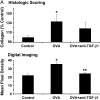
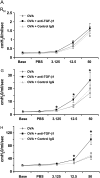
Measurements and main results: TGF-beta1 protein levels were increased in OVA-challenged lungs versus naive controls, and airway epithelial cells were shown to be a likely source of TGF-beta1. In addition, TGF-beta1 levels were elevated in OVA-exposed IL-5-null mice, which fail to recruit eosinophils into the airways. Neutralization of TGF-beta1 with specific antibody had no significant effect on airway inflammation and eosinophilia, although anti-TGF-beta1 antibody enhanced OVA-induced AHR and suppressed pulmonary fibrosis.
Conclusions: These data show that TGF-beta1 is the main TGF-beta isoform produced after OVA challenge, with a likely cellular source being the airway epithelium. The effects of blocking TGF-beta1 signaling had differential effects on AHR, fibrosis, and inflammation. While TGF-beta neutralization may be beneficial to abrogating airway remodeling, it may be detrimental to lung function by increasing AHR.
Figures



Similar articles
-
Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link?Am J Respir Cell Mol Biol. 2017 Apr;56(4):432-442. doi: 10.1165/rcmb.2016-0307TR. Am J Respir Cell Mol Biol. 2017. PMID: 27854509 Free PMC article. Review.
-
The leukotriene receptor antagonist pranlukast attenuates airway remodeling by suppressing TGF-β signaling.Pulm Pharmacol Ther. 2018 Feb;48:5-14. doi: 10.1016/j.pupt.2017.10.007. Epub 2017 Oct 13. Pulm Pharmacol Ther. 2018. PMID: 29031615
-
Surfactant protein D attenuates sub-epithelial fibrosis in allergic airways disease through TGF-β.Respir Res. 2014 Nov 29;15(1):143. doi: 10.1186/s12931-014-0143-9. Respir Res. 2014. PMID: 25472740 Free PMC article.
-
Intranasal administration of CpG oligodeoxynucleotides reduces lower airway inflammation in a murine model of combined allergic rhinitis and asthma syndrome.Int Immunopharmacol. 2015 Sep;28(1):390-8. doi: 10.1016/j.intimp.2015.06.028. Epub 2015 Jul 9. Int Immunopharmacol. 2015. PMID: 26163938
-
When engineered to produce latent TGF-beta1, antigen specific T cells down regulate Th1 cell-mediated autoimmune and Th2 cell-mediated allergic inflammatory processes.Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):89-96. doi: 10.1016/s1359-6101(99)00032-5. Cytokine Growth Factor Rev. 2000. PMID: 10708956 Review.
Cited by
-
Inhaled salmeterol and/or fluticasone alters structure/function in a murine model of allergic airways disease.Respir Res. 2010 Feb 24;11(1):22. doi: 10.1186/1465-9921-11-22. Respir Res. 2010. PMID: 20181256 Free PMC article.
-
Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-κB signaling pathway.Aging (Albany NY). 2019 Nov 6;11(21):9310-9327. doi: 10.18632/aging.102371. Epub 2019 Nov 6. Aging (Albany NY). 2019. PMID: 31692453 Free PMC article.
-
PI3K gamma-deficient mice have reduced levels of allergen-induced eosinophilic inflammation and airway remodeling.Am J Physiol Lung Cell Mol Physiol. 2009 Feb;296(2):L210-9. doi: 10.1152/ajplung.90275.2008. Epub 2008 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19028980 Free PMC article.
-
Transforming Growth Factor β1 Function in Airway Remodeling and Hyperresponsiveness. The Missing Link?Am J Respir Cell Mol Biol. 2017 Apr;56(4):432-442. doi: 10.1165/rcmb.2016-0307TR. Am J Respir Cell Mol Biol. 2017. PMID: 27854509 Free PMC article. Review.
-
Chalcone-derivative L6H21 attenuates the OVA-induced asthma by targeting MD2.Eur J Med Res. 2024 Jan 20;29(1):65. doi: 10.1186/s40001-023-01630-5. Eur J Med Res. 2024. PMID: 38245791 Free PMC article.
References
-
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827. - PubMed
-
- Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 2004;125:754–765. - PubMed
-
- Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy 2004;34:567–575. - PubMed
-
- Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997;17:326–333. - PubMed
-
- Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 2004;31:62–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

