Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation
- PMID: 17761771
- PMCID: PMC2276993
- DOI: 10.1113/jphysiol.2007.142794
Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation
Abstract
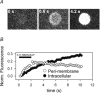
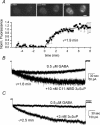
Neurosteroids are potent modulators of GABA-A receptors. We have examined the time course of development of potentiation of alpha1beta2gamma2L GABA-A receptors during coapplication of GABA and an endogenous neurosteroid (3alpha,5alpha)-3-hydroxypregnan-20-one (3alpha5alphaP). The simultaneous application of 3alpha5alphaP with 5 microm GABA resulted in a biphasic rising phase of current with time constants of 50-60 ms for the rapid phase and 0.3-3 s for the slow phase. The properties of the rapid phase were similar at all steroid concentrations but the time constant of the slower phase became successively shorter as the steroid concentration was increased. Potentiation developed very rapidly (tau = 130 ms) when cells were preincubated with 300 nm 3alpha5alphaP before application of GABA + 3alpha5alphaP, and in outside-out patch recordings, suggesting that steroid diffusion to intracellular compartments competes with receptor potentiation by depleting the cell membrane of steroid. Very low steroid concentrations (3-5 nm) potentiated GABA responses but the effects took minutes to develop. Intracellular accumulation of a fluorescent steroid analogue followed a similar time course, suggesting that slow potentiation results from slow accumulation within plasma membrane rather than indirect effects, such as activation of second messenger systems. In cell-attached single-channel recordings, where 3alpha5alphaP is normally applied through the pipette solution, addition of steroid to the bath solution dramatically shifted the steroid potentiation concentration-effect curve to lower steroid concentrations. We propose that bath-supplied steroid compensates for the diffusion of pipette-supplied steroid out of the patch to the rest of the cell membrane and/or intracellular compartments. The findings suggest that previous studies overestimate the minimum concentration of steroid capable of potentiating GABA actions at GABA-A receptors. The results have implications for the physiological role of endogenous neurosteroids.
Figures



References
-
- Akk G, Li P, Manion BD, Evers AS, Steinbach JH. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:461–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

