IP3-dependent nuclear Ca2+ signalling in the mammalian heart
- PMID: 17761776
- PMCID: PMC2277156
- DOI: 10.1113/jphysiol.2007.140731
IP3-dependent nuclear Ca2+ signalling in the mammalian heart
Abstract
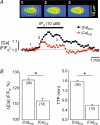
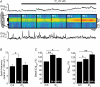
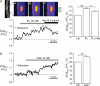
In cardiac myocytes the type-2 inositol 1,4,5-trisphosphate receptor (IP(3)R2) is the predominant isoform expressed. The IP(3)R2 channel is localized to the SR and to the nuclear envelope. We studied IP(3)-dependent nuclear Ca(2+) signals ([Ca(2+)](Nuc)) in permeabilized atrial myocytes and in isolated cardiac nuclei. In permeabilized myocytes IP(3) (20 microm) and the more potent IP(3)R agonist adenophostin (5 microm) caused an elevation of [Ca(2+)](Nuc). An IP(3)-dependent increase of [Ca(2+)](Nuc) was still observed after pretreatment with tetracaine to block Ca(2+) release from ryanodine receptors (RyRs), and the effect of IP(3) was partially reversed or prevented by the IP(3)R blockers heparin and 2-APB. Isolated nuclei were superfused with an internal solution containing the Ca(2+) indicator fluo-4 dextran. Exposure to IP(3) (10 microm) and adenophostin (0.5 microm) increased [Ca(2+)](Nuc) by 25 and 27%, respectively. [Ca(2+)](Nuc) increased to higher levels than [Ca(2+)](Cyt) immediately adjacent to the outer membrane of the nuclear envelope, suggesting that a significant portion of nuclear IP(3) receptors are facing the nucleoplasm. When nuclei were pretreated with heparin or 2-APB, IP(3) failed to increase [Ca(2+)](Nuc). Isolated nuclei were also loaded with the membrane-permeant low-affinity Ca(2+) probe fluo-5N AM which compartmentalized into the nuclear envelope. Exposure to IP(3) and adenophostin resulted in a decrease of the fluo-5N signal that could be prevented by heparin. Stimulation of IP(3)R caused depletion of the nuclear Ca(2+) stores by approximately 60% relative to the maximum depletion produced by the ionophores ionomycin and A23187. The fluo-5N fluorescence decrease was particularly pronounced in the nuclear periphery, suggesting that the nuclear envelope may represent the predominant nuclear Ca(2+) store. The data indicate that IP(3) can elicit Ca(2+) release from cardiac nuclei resulting in localized nuclear Ca(2+) signals.
Figures
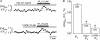



Similar articles
-
Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias.J Physiol. 2004 Mar 16;555(Pt 3):607-15. doi: 10.1113/jphysiol.2003.058529. Epub 2004 Jan 30. J Physiol. 2004. PMID: 14754996 Free PMC article.
-
[Investigation of nuclear Ca2+ regulation in the isolated cardiac nuclei].Shi Yan Sheng Wu Xue Bao. 2002 Jun;35(2):127-34. Shi Yan Sheng Wu Xue Bao. 2002. PMID: 15344331 Chinese.
-
IP3-induced cytosolic and nuclear Ca2+ signals in HL-1 atrial myocytes: possible role of IP3 receptor subtypes.Mol Cells. 2010 Apr;29(4):387-95. doi: 10.1007/s10059-010-0039-6. Epub 2010 Mar 4. Mol Cells. 2010. PMID: 20213315
-
Atrial local Ca2+ signaling and inositol 1,4,5-trisphosphate receptors.Prog Biophys Mol Biol. 2010 Sep;103(1):59-70. doi: 10.1016/j.pbiomolbio.2010.02.002. Epub 2010 Mar 1. Prog Biophys Mol Biol. 2010. PMID: 20193706 Review.
-
Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes.J Mol Cell Cardiol. 2008 Aug;45(2):128-47. doi: 10.1016/j.yjmcc.2008.05.014. Epub 2008 Jun 15. J Mol Cell Cardiol. 2008. PMID: 18603259 Free PMC article. Review.
Cited by
-
Calcium signalling in developing cardiomyocytes: implications for model systems and disease.J Physiol. 2015 Mar 1;593(5):1047-63. doi: 10.1113/jphysiol.2014.274712. Epub 2015 Feb 9. J Physiol. 2015. PMID: 25641733 Free PMC article. Review.
-
Activation of NFATc1 is directly mediated by IP3 in adult cardiac myocytes.Am J Physiol Heart Circ Physiol. 2010 Nov;299(5):H1701-7. doi: 10.1152/ajpheart.00470.2010. Epub 2010 Sep 17. Am J Physiol Heart Circ Physiol. 2010. PMID: 20852052 Free PMC article.
-
Nuclear Calcium in Cardiac (Patho)Physiology: Small Compartment, Big Impact.Biomedicines. 2023 Mar 21;11(3):960. doi: 10.3390/biomedicines11030960. Biomedicines. 2023. PMID: 36979939 Free PMC article. Review.
-
Role of inositol 1,4,5-trisphosphate in the regulation of ventricular Ca(2+) signaling in intact mouse heart.J Mol Cell Cardiol. 2012 Dec;53(6):768-79. doi: 10.1016/j.yjmcc.2012.08.019. Epub 2012 Aug 31. J Mol Cell Cardiol. 2012. PMID: 22960455 Free PMC article.
-
SERCA Activity Controls the Systolic Calcium Increase in the Nucleus of Cardiac Myocytes.Front Physiol. 2019 Feb 6;10:56. doi: 10.3389/fphys.2019.00056. eCollection 2019. Front Physiol. 2019. PMID: 30787882 Free PMC article.
References
-
- Bading H, Hardingham GE, Johnson CM, Chawla S. Gene regulation by nuclear and cytoplasmic calcium signals. Biochem Biophys Res Commun. 1997;236:541–543. - PubMed
-
- Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:15912–15920. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Blatter LA, Wier WG. Agonist-induced [Ca2+]i waves and Ca2+-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1992;263:H576–H586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

