Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging
- PMID: 17761778
- PMCID: PMC2276997
- DOI: 10.1113/jphysiol.2007.134338
Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging
Abstract
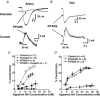
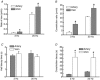
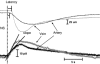
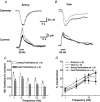
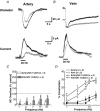
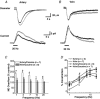
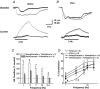
As arteries are resistance blood vessels while veins perform a capacitance function, it might be expected that sympathetic neural control of arteries and veins would differ. The function of sympathetic nerves supplying mesenteric arteries (MA) and veins (MV) in rats was investigated using in vitro continuous amperometry with a carbon fibre microelectrode and video imaging. We simultaneously measured noradrenaline (NA) overflow at the blood vessel adventitial surface and vasoconstriction evoked by electrical stimulation of perivascular sympathetic nerves. Sympathetic nerve arrangement was studied using glyoxylic acid-induced fluorescence of NA. We found that: (i) there were significant differences between MA and MV in the arrangement of sympathetic nerves; (ii) frequency-response curves for NA overflow and vasoconstriction for MV were left-shifted compared to MA; (iii) the P2X receptor antagonist, pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid (PPADS, 10 microm), reduced constrictions in MA but not in MV while the alpha(1)-adrenergic receptor antagonist, prazosin (0.1 microm), blocked constrictions in MV but not in MA; (iv) NA overflow for MA was enhanced by the alpha(2)-adrenergic receptor antagonist, yohimbine (1.0 microm), and attenuated by the alpha(2)-adrenergic receptor agonist, UK 14,304 (1.0 microm), while yohimbine and UK 14,304 had little effect in MV; (v) cocaine (10 microm) produced larger increases in NA overflow in MA than in MV; (vi) UK 14,304 constricted MV but not MA while yohimbine reduced constrictions in MV but not MA. We conclude that there are fundamental differences in sympathetic neuroeffector mechanisms in MA and MV, which are likely to contribute to their different haemodynamic functions.
Figures


Similar articles
-
Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension.Auton Neurosci. 2010 Jan 15;152(1-2):11-20. doi: 10.1016/j.autneu.2009.08.008. Epub 2009 Nov 13. Auton Neurosci. 2010. PMID: 19914150 Free PMC article.
-
Cotransmission from sympathetic vasoconstrictor neurons: differences in guinea-pig mesenteric artery and vein.Auton Neurosci. 2000 Dec 28;86(1-2):18-29. doi: 10.1016/S1566-0702(00)00203-4. Auton Neurosci. 2000. PMID: 11269921
-
Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats.Auton Neurosci. 2003 Feb 28;104(1):47-57. doi: 10.1016/S1566-0702(02)00287-4. Auton Neurosci. 2003. PMID: 12559203
-
Presynaptic alpha2-adrenoceptor-mediated modulation of adenosine 5' triphosphate and noradrenaline corelease: differences in canine mesenteric artery and vein.J Auton Pharmacol. 2001 Feb;21(1):47-55. doi: 10.1046/j.1365-2680.2001.00207.x. J Auton Pharmacol. 2001. PMID: 11422578
-
Properties of the venous and arterial innervation in the mesentery.J Smooth Muscle Res. 2003 Dec;39(6):269-79. doi: 10.1540/jsmr.39.269. J Smooth Muscle Res. 2003. PMID: 15048018 Review. No abstract available.
Cited by
-
Spinal cord injury alters purinergic neurotransmission to mesenteric arteries in rats.Am J Physiol Heart Circ Physiol. 2020 Feb 1;318(2):H223-H237. doi: 10.1152/ajpheart.00525.2019. Epub 2019 Nov 27. Am J Physiol Heart Circ Physiol. 2020. PMID: 31774690 Free PMC article.
-
In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell.Neuroscience. 2010 Aug 11;169(1):132-42. doi: 10.1016/j.neuroscience.2010.04.076. Epub 2010 May 6. Neuroscience. 2010. PMID: 20451589 Free PMC article.
-
Naloxone selectively inhibits vasoconstriction caused by phenylephrine but not endogenous noradrenaline in the rat mesenteric vasculature.J Smooth Muscle Res. 2024;60:54-63. doi: 10.1540/jsmr.60.54. J Smooth Muscle Res. 2024. PMID: 39567020 Free PMC article.
-
Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension.Auton Neurosci. 2010 Jan 15;152(1-2):11-20. doi: 10.1016/j.autneu.2009.08.008. Epub 2009 Nov 13. Auton Neurosci. 2010. PMID: 19914150 Free PMC article.
-
Vascular impairment of adenosinergic system in hypertension: increased adenosine bioavailability and differential distribution of adenosine receptors and nucleoside transporters.Histochem Cell Biol. 2019 May;151(5):407-418. doi: 10.1007/s00418-018-1743-0. Epub 2018 Oct 24. Histochem Cell Biol. 2019. PMID: 30357508
References
-
- Bjorklund A, Lindvall O, Svensson LA. Mechanisms of fluorophore formation in the histochemical glyoxylic acid method for monoamines. Histochemie. 1972;32:113–131. - PubMed
-
- Bobalova J, Mutafova-Yambolieva VN. Presynaptic α2-adrenoceptor-mediated modulation of adenosine 5′-triphosphate and noradrenaline corelease: differences in canine mesenteric artery and vein. J Auton Pharmacol. 2001;21:47–55. - PubMed
-
- Browning K, Zheng Z, Kreulen D, Travagli R. Two populations of sympathetic neurons project selectively to mesenteric artery or vein. Am J Physiol Heart Circ Physiol. 1999;276:H1263–H1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

