A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid
- PMID: 17761794
- PMCID: PMC1964862
- DOI: 10.1073/pnas.0705459104
A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid
Abstract
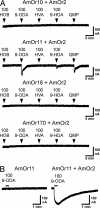
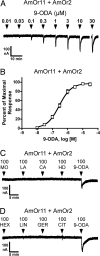
By using a functional genomics approach, we have identified a honey bee [Apis mellifera (Am)] odorant receptor (Or) for the queen substance 9-oxo-2-decenoic acid (9-ODA). Honey bees live in large eusocial colonies in which a single queen is responsible for reproduction, several thousand sterile female worker bees complete a myriad of tasks to maintain the colony, and several hundred male drones exist only to mate. The "queen substance" [also termed the queen retinue pheromone (QRP)] is an eight-component pheromone that maintains the queen's dominance in the colony. The main component, 9-ODA, acts as a releaser pheromone by attracting workers to the queen and as a primer pheromone by physiologically inhibiting worker ovary development; it also acts as a sex pheromone, attracting drones during mating flights. However, the extent to which social and sexual chemical messages are shared remains unresolved. By using a custom chemosensory-specific microarray and qPCR, we identified four candidate sex pheromone Ors (AmOr10, -11, -18, and -170) from the honey bee genome based on their biased expression in drone antennae. We assayed the pheromone responsiveness of these receptors by using Xenopus oocytes and electrophysiology. AmOr11 responded specifically to 9-ODA (EC50=280+/-31 nM) and not to any of the other seven QRP components, other social pheromones, or floral odors. We did not observe any responses of the other three Ors to any of the eight QRP pheromone components, suggesting 9-ODA is the only QRP component that also acts as a long-distance sex pheromone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Crespi BJ, Yanega D. Behav Ecol. 1995;6:109–115.
-
- Winston ML. The Biology of the Honeybee. Cambridge, MA: Harvard Univ Press; 1987.
-
- Slessor KN, Winston ML, Le Conte Y. J Chem Ecol. 2005;31:2731–2745. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

