Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis
- PMID: 17761833
- PMCID: PMC2234798
- DOI: 10.1182/blood-2006-11-057299
Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis
Abstract
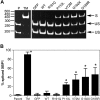
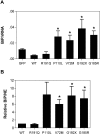
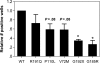
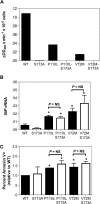
Severe congenital neutropenia (SCN) is an inborn disorder of granulopoiesis. Mutations of the ELA2 gene encoding neutrophil elastase (NE) are responsible for most cases of SCN and cyclic neutropenia (CN), a related but milder disorder of granulopoiesis. However, the mechanisms by which these mutations disrupt granulopoiesis are unclear. We hypothesize that the ELA2 mutations result in the production of misfolded NE protein, activation of the unfolded protein response (UPR), and ultimately apoptosis of granulocytic precursors. Expression of mutant NE but not wild-type NE strongly induced BiP/GRP78 mRNA expression and XBP1 mRNA splicing, 2 classic markers of the UPR. The magnitude of UPR activation by a specific ELA2 mutation correlated with its associated clinical phenotype. Consistent with the UPR model, expression of mutant NE in primary human granulocytic precursors increased expression of CHOP (DDITS) and induced apoptosis in a protease-independent fashion. Most strikingly, UPR activation and decreased NE protein expression were detected in primary granulocytic precursors from SCN patients. Collectively, these data provide strong support for a UPR model of SCN disease pathogenesis and place SCN in a growing list of human diseases caused by misfolded proteins.
Figures

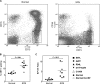
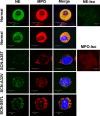
References
-
- Kostmann R. Infantile genetic agranulocytosis: a new recessive lethal disease in man. Acta Paediatr. 1956;105:1–78. - PubMed
-
- Welte K, Zeidler C, Dale DC. Severe congenital neutropenia. Semin Hematol. 2006;43:189–195. - PubMed
-
- Haurie C, Dale DC, Mackey MC. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood. 1998;92:2629–2640. - PubMed
-
- Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic hematopoiesis. Nat Genet. 1999;23:433–436. - PubMed
-
- Ancliff PJ, Gale RE, Liesner R, Hann IM, Linch DC. Mutations in the ELA2 gene encoding neutrophil elastase are present in most patients with sporadic severe congenital neutropenia but only in some patients with the familial form of the disease. Blood. 2001;98:2645–2650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

