Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation
- PMID: 17761939
- PMCID: PMC2376762
- DOI: 10.1161/ATVBAHA.107.151100
Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation
Abstract
Objective: Platelets play a dual role in thrombosis by forming aggregates and stimulating coagulation. We investigated the commitment of platelets to these separate functions during collagen-induced thrombus formation in vitro and in vivo.
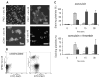
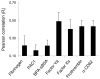
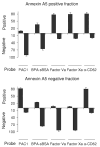
Methods and results: High-resolution 2-photon fluorescence microscopy revealed that in thrombus formation under flow, fibrin(ogen)-binding platelets assembled into separate aggregates, whereas distinct patches of nonaggregated platelets exposed phosphatidylserine. The latter platelet population had inactivated alphaIIb beta3 integrins and displayed increased binding of coagulation factors. Coated platelets, expressing serotonin binding sites, were not identified as a separate population. Thrombin generation and coagulation favored the transformation to phosphatidylserine-exposing platelets with inactivated integrins and reduced adhesion. Prolonged tyrosine phosphorylation in vitro resulted in secondary downregulation of active alphaIIb beta3.
Conclusions: These results lead to a new spatial model of thrombus formation, in which aggregated platelets ensure thrombus stability, whereas distinct patches of nonaggregated platelets effectuate procoagulant activity and generate thrombin and fibrin. Herein, the hemostatic activity of a developing thrombus is determined by the balance in formation of proaggregatory and procoagulant platelets. This balance is influenced by antiplatelet and anticoagulant medication.
Figures

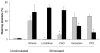
References
-
- Heemskerk JWM, Kuijpers MJE, Munnix ICA, Siljander PRM. Platelet collagen receptors and coagulation. Trends Cardiovasc Med. 2005;15:86–92. - PubMed
-
- Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid assymetry in blood cells. Blood. 1997;89:1121–1132. - PubMed
-
- Munnix ICA, Harmsma M, Giddings JC, Collins PW, Feijge MAH, Comfurius P, Heemskerk JWM, Bevers EM. Store-mediated calcium entry in the regulation of phosphatidylserine exposure in blood cells from Scott patients. Thromb Hemost. 2003;89:687–695. - PubMed
-
- Butenas S, van ’t Veer C, Mann KG. ‘Normal’ thrombin generation. Blood. 1999;94:2169–2178. - PubMed
-
- Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–1389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

