The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha
- PMID: 17761946
- PMCID: PMC2668600
- DOI: 10.1210/me.2007-0293
The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha
Abstract
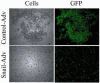
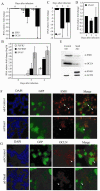
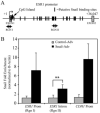
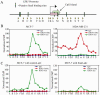
The estrogen receptor (ER)-alpha (ESR1) is a key regulatory molecule in mammary epithelial cell development. Loss of ER-alpha in breast cancer is correlated with poor prognosis, increased recurrence after treatment, and an elevated incidence of metastasis. A proposed molecular pathway by which ER-alpha acts to constrain invasive growth in breast cancer cells involves direct, ER-alpha-dependent expression of metastasis-associated protein 3, a cell-type-specific component of the Mi-2/NuRD chromatin remodeling complex. MTA3 in turn represses expression of Snail, a transcription factor linked to epithelial to mesenchymal transition and cancer metastasis. To elucidate its role(s) in epithelial to mesenchymal transition (EMT), we expressed Snail in the noninvasive, ER-alpha-positive MCF-7 cell line. Snail expression led to decreased cell-cell adhesion and increased cell invasiveness. Furthermore, we observed loss of ER-alpha expression at both the RNA and protein level that was accompanied by direct interaction of Snail with regulatory DNA sequences at the ESR1 locus. A consequence of loss of ER-alpha function in this system was the increased abundance of key components of the TGF-beta signaling pathway. Thus, cross-talk among ER-alpha, Snail, and the TGF-beta pathway appears to control critical phenotypic properties of breast cancer cells.
Figures



References
-
- Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. - PubMed
-
- Ferguson AT, Lapidus RG, Davidson NE. The regulation of estrogen receptor expression and function in human breast cancer. Cancer Treat Res. 1998;94:255–278. - PubMed
-
- Prall OW, Rogan EM, Sutherland RL. Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol. 1998;65:169–174. - PubMed
-
- Clarke RB, Anderson E, Howell A. Steroid receptors in human breast cancer. Trends Endocrinol Metab. 2004;15:316–323. - PubMed
-
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

