Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions
- PMID: 17762862
- PMCID: PMC2230676
- DOI: 10.1038/sj.emboj.7601841
Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions
Abstract
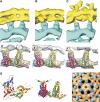
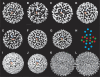
Hepatitis B virus (HBV) is a major human pathogen causing about 750,000 deaths per year. The virion consists of a nucleocapsid and an envelope formed by lipids, and three integral membrane proteins. Although we have detailed structural insights into the organization of the HBV core, the arrangement of the envelope in virions and its interaction with the nucleocapsid is elusive. Here we show the ultrastructure of hepatitis B virions purified from patient serum. We identified two morphological phenotypes, which appear as compact and gapped particles with nucleocapsids in distinguishable conformations. The overall structures of these nucleocapsids resemble recombinant cores with two alpha-helical spikes per asymmetric unit. At the charged tips the spikes are contacted by defined protrusions of the envelope proteins, probably via electrostatic interactions. The HBV envelope in the two morphotypes is to some extent variable, but the surface proteins follow a general packing scheme with up to three surface protein dimers per asymmetric unit. The variability in the structure of the envelope indicates that the nucleocapsid does not firmly constrain the arrangement of the surface proteins, but provides a general template for the packing.
Figures


References
-
- Böttcher B, Vogel M, Ploss M, Nassal M (2006) High plasticity of the hepatitis B virus capsid revealed by conformational stress. J Mol Biol 356: 812–822 - PubMed
-
- Böttcher B, Wynne SA, Crowther RA (1997) Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 386: 88–91 - PubMed
-
- Bruss V (2004) Envelopment of the hepatitis B virus nucleocapsid. Virus Res 106: 199–209 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

