Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas
- PMID: 17764010
- PMCID: PMC11030195
- DOI: 10.1007/s00262-007-0391-3
Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas
Abstract
Introduction: Vgamma9Vdelta2 T lymphocytes are reported to participate in the anti-tumor immune surveillance in human. They are known to recognize phosphoantigens and molecules expressed on cells undergoing neoplasic transformation. In this study, we investigated phenotype and anti-tumor cytotoxicity of ex vivo expanded Vgamma9Vdelta2 T cells in view of adoptive immunotherapy.
Materials and methods: Experiments were performed with peripheral blood samples from eleven patients [six colorectal carcinoma (CRC), four hepatocellular carcinoma (HCC), one sarcoma] and sixteen healthy donors.
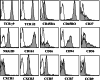
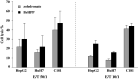
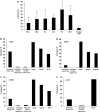
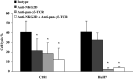
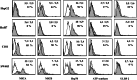
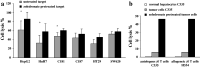
Results/discussion: Ex vivo expansion of Vgamma9Vdelta2 T cells could be achieved by a single dose of phosphoantigen, either bromohydrin pyrophosphate or zoledronate, and supported by exogenous IL-2. After 2 weeks, expanded Vgamma9Vdelta2 T lymphocytes acquired the effector memory phenotype CD45RA(-)CD45RO(high)CD27(-). They expressed NKG2D and CD161 and the proinflammatory CXCR3 and CCR5 chemokine receptors. Vgamma9Vdelta2 T cells displayed a strong lytic activity toward a broad panel of tumor cell lines or primary cultures. Interestingly, HCC and CRC primary cells could be lysed by autologous Vgamma9Vdelta2 T cells whereas autologous normal cells were not sensitive to the lysis. mAbs blocking assays demonstrated that TCR was the most important receptor involved in the lysis of tumor cells. However, NKG2D receptor could deliver a costimulatory signal enhancing the lysis of HCC and CRC tumors expressing MICA/B. Treatment of tumor cells by the mevalonate pathway inhibitor, zoledronate, enhanced the killing of both HCC and CRC. Expansion index of Vgamma9Vdelta2 T cells was in similar levels in healthy donors or in cancer patients and total expansion was suitable for adoptive immunotherapy.
Conclusion: These results provide a rationale for the clinical evaluation of Vgamma9Vdelta2 T lymphocytes in HCC and CRC.
Figures

References
-
- Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–7795. - PubMed
-
- Barkholt L, Danielsson R, Calissendorff B, Svensson L, Malihi R, Remberger M, Uzunel M, Thorne A, Ringden O. Indium-111-labelled donor-lymphocyte infusion by way of hepatic artery and radio-frequency ablation against liver metastases of renal and colon carcinoma after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78:697–703. doi: 10.1097/01.TP.0000129807.53523.97. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

