Separation of decay-accelerating and cofactor functional activities of Kaposi's sarcoma-associated herpesvirus complement control protein using monoclonal antibodies
- PMID: 17764451
- PMCID: PMC2433302
- DOI: 10.1111/j.1365-2567.2007.02692.x
Separation of decay-accelerating and cofactor functional activities of Kaposi's sarcoma-associated herpesvirus complement control protein using monoclonal antibodies
Abstract
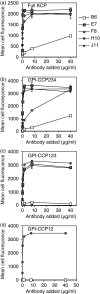
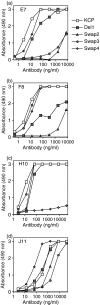
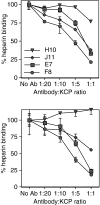
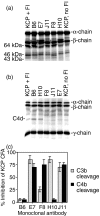
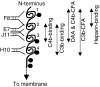
Complement is an essential part of the innate immune system, which clears pathogens without requirement for previous exposure, although it also greatly enhances the efficacy and response of the cellular and humoral immune systems. Kaposi's sarcoma-associated herpesvirus (KSHV) is the most recently identified human herpesvirus and the likely aetiological agent of Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease. We previously reported that the KSHV complement control protein (KCP) was expressed on infected cells and virions, and could inhibit complement through decay-accelerating activity (DAA) of the classical C3 convertase and cofactor activity (CFA) for factor I (FI)-mediated degradation of C4b and C3b, as well as acting as an attachment factor for binding to heparan sulphate on permissive cells. Here, we determined the ability of a panel of monoclonal anti-KCP antibodies to block KCP functions relative to their recognized epitopes, as determined through binding to recombinant KCP containing large (entire domain) or small (2-3 amino acid residue) alterations. One antibody recognizing complement control protein (CCP) domain 1 blocked heparin binding, DAA and C4b CFA, but was poor at blocking C3b CFA, while a second antibody recognizing CCP4 blocked C3b CFA and 80% DAA, but not C4b CFA or heparan sulphate binding. Two antibodies recognizing CCP2 and CCP3 were capable of blocking C3b and C4b CFA and heparan sulphate binding, but only one could inhibit DAA. These results show that, while KCP is a multifunctional protein, these activities do not completely overlap and can be isolated through incubation with monoclonal antibodies.
Figures
Similar articles
-
Dissecting the regions of virion-associated Kaposi's sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding.J Virol. 2006 Apr;80(8):4068-78. doi: 10.1128/JVI.80.8.4068-4078.2006. J Virol. 2006. PMID: 16571823 Free PMC article.
-
The Kaposi's sarcoma-associated herpesvirus complement control protein mimics human molecular mechanisms for inhibition of the complement system.J Biol Chem. 2004 Oct 22;279(43):45093-101. doi: 10.1074/jbc.M407558200. Epub 2004 Aug 10. J Biol Chem. 2004. PMID: 15304516
-
Identification of functional domains in kaposica, the complement control protein homolog of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8).J Virol. 2005 May;79(9):5850-6. doi: 10.1128/JVI.79.9.5850-5856.2005. J Virol. 2005. PMID: 15827200 Free PMC article.
-
Kaposi's sarcoma-associated herpes virus complement control protein: KCP--complement inhibition and more.Mol Immunol. 2007 Jan;44(1-3):11-22. doi: 10.1016/j.molimm.2006.06.018. Epub 2006 Aug 14. Mol Immunol. 2007. PMID: 16905191 Review.
-
Modulation of the immune system by Kaposi's sarcoma-associated herpesvirus.Trends Microbiol. 2009 Mar;17(3):119-29. doi: 10.1016/j.tim.2008.12.001. Epub 2009 Feb 18. Trends Microbiol. 2009. PMID: 19230674 Review.
Cited by
-
Modulation of Immune System by Kaposi's Sarcoma-Associated Herpesvirus: Lessons from Viral Evasion Strategies.Front Microbiol. 2012 Mar 5;3:44. doi: 10.3389/fmicb.2012.00044. eCollection 2012. Front Microbiol. 2012. PMID: 22403573 Free PMC article.
-
Viral-derived complement inhibitors: current status and potential role in immunomodulation.Exp Biol Med (Maywood). 2017 Feb;242(4):397-410. doi: 10.1177/1535370216675772. Epub 2016 Oct 26. Exp Biol Med (Maywood). 2017. PMID: 27798122 Free PMC article. Review.
-
Molecular biology of KSHV lytic reactivation.Viruses. 2015 Jan 14;7(1):116-53. doi: 10.3390/v7010116. Viruses. 2015. PMID: 25594835 Free PMC article. Review.
-
KSHV: pathways to tumorigenesis and persistent infection.Adv Virus Res. 2014;88:111-59. doi: 10.1016/B978-0-12-800098-4.00002-7. Adv Virus Res. 2014. PMID: 24373311 Free PMC article. Review.
-
Immunization of Mice with Virus-Like Vesicles of Kaposi Sarcoma-Associated Herpesvirus Reveals a Role for Antibodies Targeting ORF4 in Activating Complement-Mediated Neutralization.J Virol. 2023 Feb 28;97(2):e0160022. doi: 10.1128/jvi.01600-22. Epub 2023 Feb 9. J Virol. 2023. PMID: 36757205 Free PMC article.
References
-
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. - PubMed
-
- Watanabe I, Ross TM, Tamura S, et al. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine. 2003;21:4532–8. - PubMed
-
- Bower JF, Green TD, Ross TM. DNA vaccines expressing soluble CD4-envelope proteins fused to C3d elicit cross-reactive neutralizing antibodies to HIV-1. Virology. 2004;328:292–300. - PubMed
-
- Kirkitadze MD, Barlow PN. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol Rev. 2001;180:146–61. - PubMed
-
- Norman DG, Barlow PN, Baron M, Day AJ, Sim RB, Campbell ID. Three-dimensional structure of a complement control protein module in solution. J Mol Biol. 1991;219:717–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

