IFN-gamma amplifies NFkappaB-dependent Neisseria meningitidis invasion of epithelial cells via specific upregulation of CEA-related cell adhesion molecule 1
- PMID: 17764466
- PMCID: PMC3020365
- DOI: 10.1111/j.1462-5822.2007.01038.x
IFN-gamma amplifies NFkappaB-dependent Neisseria meningitidis invasion of epithelial cells via specific upregulation of CEA-related cell adhesion molecule 1
Abstract
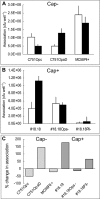
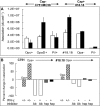
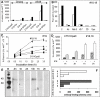
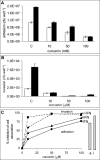
Temporal relationship between viral and bacterial infections has been observed, and may arise via the action of virus-induced inflammatory cytokines. These, by upregulating epithelial receptors targeted by bacteria, may encourage greater bacterial infiltration. In this study, human epithelial cells exposed to interferon-gamma but not tumour necrosis factor-alpha or interleukin 1-beta supported increased meningococcal adhesion and invasion. The increase was related to Opa but not Opc or pili adhesin expression. De novo synthesis of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), a major Opa receptor, occurred in epithelial cells exposed to the cytokine, or when infected with Opa-expressing bacteria. Cell line-dependent differences in invasion that were observed could be correlated with CEACAM expression levels. There was also evidence for Opa/pili synergism leading to high levels of monolayer infiltration by capsulate bacteria. The use of nuclear factor-kappa B (NFkappaB) inhibitors, diferuloylmethane (curcumin) and SN50, abrogated bacterial infiltration of both untreated and interferon-gamma-treated cells. The studies demonstrate the importance of CEACAMs as mediators of increased cellular invasion under conditions of inflammation and bring to light the potential role of NFkappaB pathway in Opa-mediated invasion by meningococci. The data imply that cell-surface remodelling by virally induced cytokines could be one factor that increases host susceptibility to bacterial infection.
Figures





Similar articles
-
Co-ordinate action of bacterial adhesins and human carcinoembryonic antigen receptors in enhanced cellular invasion by capsulate serum resistant Neisseria meningitidis.Cell Microbiol. 2007 Jan;9(1):154-68. doi: 10.1111/j.1462-5822.2006.00775.x. Epub 2006 Aug 2. Cell Microbiol. 2007. PMID: 16889622
-
Critical determinants of the interactions of capsule-expressing Neisseria meningitidis with host cells: the role of receptor density in increased cellular targeting via the outer membrane Opa proteins.Cell Microbiol. 2005 Oct;7(10):1490-503. doi: 10.1111/j.1462-5822.2005.00572.x. Cell Microbiol. 2005. PMID: 16153247
-
Neisseria meningitidis has two independent modes of recognizing its human receptor CEACAM1.PLoS One. 2011 Jan 27;6(1):e14609. doi: 10.1371/journal.pone.0014609. PLoS One. 2011. PMID: 21298042 Free PMC article.
-
Meningococcal interactions with the host.Vaccine. 2009 Jun 24;27 Suppl 2:B78-89. doi: 10.1016/j.vaccine.2009.04.069. Epub 2009 May 28. Vaccine. 2009. PMID: 19481311 Review.
-
Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria.FEMS Microbiol Rev. 2011 May;35(3):498-514. doi: 10.1111/j.1574-6976.2010.00260.x. Epub 2011 Jan 17. FEMS Microbiol Rev. 2011. PMID: 21204865 Review.
Cited by
-
A novel group of Moraxella catarrhalis UspA proteins mediates cellular adhesion via CEACAMs and vitronectin.PLoS One. 2012;7(9):e45452. doi: 10.1371/journal.pone.0045452. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049802 Free PMC article.
-
Echovirus-30 Infection Alters Host Proteins in Lipid Rafts at the Cerebrospinal Fluid Barrier In Vitro.Microorganisms. 2020 Dec 10;8(12):1958. doi: 10.3390/microorganisms8121958. Microorganisms. 2020. PMID: 33321840 Free PMC article.
-
The Moraxella adhesin UspA1 binds to its human CEACAM1 receptor by a deformable trimeric coiled-coil.EMBO J. 2008 Jun 18;27(12):1779-89. doi: 10.1038/emboj.2008.101. Epub 2008 May 22. EMBO J. 2008. PMID: 18497748 Free PMC article.
-
RNA-Seq-based analysis of the physiologic cold shock-induced changes in Moraxella catarrhalis gene expression.PLoS One. 2013 Jul 2;8(7):e68298. doi: 10.1371/journal.pone.0068298. Print 2013. PLoS One. 2013. PMID: 23844181 Free PMC article.
-
The E-Cadherin Cleavage Associated to Pathogenic Bacteria Infections Can Favor Bacterial Invasion and Transmigration, Dysregulation of the Immune Response and Cancer Induction in Humans.Front Microbiol. 2019 Nov 8;10:2598. doi: 10.3389/fmicb.2019.02598. eCollection 2019. Front Microbiol. 2019. PMID: 31781079 Free PMC article. Review.
References
-
- Aho EL, Dempsey JA, Hobbs MM, Klapper DG, Cannon JG. Characterization of the opa (class 5) gene family of Neisseria meningitidis. Mol Microbiol. 1991;5:1429–1437. - PubMed
-
- Alonso JM, Guiyoule A, Zarantonelli ML, Ramisse F, Pires R, Antignac A, et al. A model of meningococcal bacteremia after respiratory superinfection in influenza a virus-infected mice. FEMS Microbiol Lett. 2003;222:99–106. - PubMed
-
- Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17:185–191. - PubMed
-
- Bhat KS, Gibbs CP, Barrera O, Morrison SG, Jahnig F, Stern A, et al. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

