The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants
- PMID: 17764515
- PMCID: PMC2265002
- DOI: 10.1111/j.1365-313X.2007.03259.x
The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants
Abstract
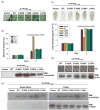
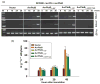
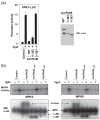
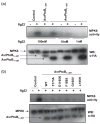
Resistance to bacterial speck disease in tomato is activated by the physical interaction of the host Pto kinase with either of the sequence-dissimilar type III effector proteins AvrPto or AvrPtoB (HopAB2) from Pseudomonas syringae pv. tomato. Pto-mediated immunity requires Prf, a protein with a nucleotide-binding site and leucine-rich repeats. The N-terminal 307 amino acids of AvrPtoB were previously reported to interact with the Pto kinase, and we show here that this region (AvrPtoB(1-307)) is sufficient for eliciting Pto/Prf-dependent immunity against P. s. pv. tomato. AvrPtoB(1-307) was also found to be sufficient for a virulence activity that enhances ethylene production and increases growth of P. s. pv. tomato and severity of speck disease on susceptible tomato lines lacking either Pto or Prf. Moreover, we found that residues 308-387 of AvrPtoB are required for the previously reported ability of AvrPtoB to suppress pathogen-associated molecular patterns-induced basal defenses in Arabidopsis. Thus, the N-terminal region of AvrPtoB has two structurally distinct domains involved in different virulence-promoting mechanisms. Random and targeted mutagenesis identified five tightly clustered residues in AvrPtoB(1-307) that are required for interaction with Pto and for elicitation of immunity to P. s. pv. tomato. Mutation of one of the five clustered residues abolished the ethylene-associated virulence activity of AvrPtoB(1-307). However, individual mutations of the other four residues, despite abolishing interaction with Pto and avirulence activity, had no effect on AvrPtoB(1-307) virulence activity. None of these mutations affected the basal defense-suppressing activity of AvrPtoB(1-387). Based on sequence alignments, estimates of helical propensity, and the previously reported structure of AvrPto, we hypothesize that the Pto-interacting domains of AvrPto and AvrPtoB(1-307) have structural similarity. Together, these data support a model in which AvrPtoB(1-307) promotes ethylene-associated virulence by interaction not with Pto but with another unknown host protein.
Figures





Similar articles
-
Diverse AvrPtoB homologs from several Pseudomonas syringae pathovars elicit Pto-dependent resistance and have similar virulence activities.Appl Environ Microbiol. 2006 Jan;72(1):702-12. doi: 10.1128/AEM.72.1.702-712.2006. Appl Environ Microbiol. 2006. PMID: 16391110 Free PMC article.
-
Pto- and Prf-mediated recognition of AvrPto and AvrPtoB restricts the ability of diverse pseudomonas syringae pathovars to infect tomato.Mol Plant Microbe Interact. 2007 Jul;20(7):806-15. doi: 10.1094/MPMI-20-7-0806. Mol Plant Microbe Interact. 2007. PMID: 17601168
-
Pseudomonas syringae type III effector AvrPtoB is phosphorylated in plant cells on serine 258, promoting its virulence activity.J Biol Chem. 2007 Oct 19;282(42):30737-44. doi: 10.1074/jbc.M705565200. Epub 2007 Aug 20. J Biol Chem. 2007. PMID: 17711844 Free PMC article.
-
AvrPtoB: a bacterial type III effector that both elicits and suppresses programmed cell death associated with plant immunity.FEMS Microbiol Lett. 2005 Apr 1;245(1):1-8. doi: 10.1016/j.femsle.2005.02.025. FEMS Microbiol Lett. 2005. PMID: 15796972 Review.
-
Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato.Annu Rev Phytopathol. 2003;41:215-43. doi: 10.1146/annurev.phyto.41.121602.143032. Annu Rev Phytopathol. 2003. PMID: 14527329 Review.
Cited by
-
The tomato calcium sensor Cbl10 and its interacting protein kinase Cipk6 define a signaling pathway in plant immunity.Plant Cell. 2013 Jul;25(7):2748-64. doi: 10.1105/tpc.113.113530. Epub 2013 Jul 31. Plant Cell. 2013. PMID: 23903322 Free PMC article.
-
Bypassing kinase activity of the tomato Pto resistance protein with small molecule ligands.J Biol Chem. 2009 May 29;284(22):15289-98. doi: 10.1074/jbc.M809724200. Epub 2009 Mar 30. J Biol Chem. 2009. PMID: 19332544 Free PMC article.
-
Endosome-associated CRT1 functions early in resistance gene-mediated defense signaling in Arabidopsis and tobacco.Plant Cell. 2010 Mar;22(3):918-36. doi: 10.1105/tpc.109.071662. Epub 2010 Mar 23. Plant Cell. 2010. PMID: 20332379 Free PMC article.
-
Behind the lines-actions of bacterial type III effector proteins in plant cells.FEMS Microbiol Rev. 2016 Nov 1;40(6):894-937. doi: 10.1093/femsre/fuw026. FEMS Microbiol Rev. 2016. PMID: 28201715 Free PMC article. Review.
-
The potato cyst nematode effector RHA1B is a ubiquitin ligase and uses two distinct mechanisms to suppress plant immune signaling.PLoS Pathog. 2019 Apr 12;15(4):e1007720. doi: 10.1371/journal.ppat.1007720. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30978251 Free PMC article.
References
-
- Abramovitch RB, Martin GB. Strategies used by bacterial pathogens to suppress plant defenses. Curr Opin Plant Biol. 2004;7:356–364. - PubMed
-
- Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol. 2004;42:385–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

