Epstein-Barr virus latency switch in human B-cells: a physico-chemical model
- PMID: 17764547
- PMCID: PMC2164963
- DOI: 10.1186/1752-0509-1-40
Epstein-Barr virus latency switch in human B-cells: a physico-chemical model
Abstract
Background: The Epstein-Barr virus is widespread in all human populations and is strongly associated with human disease, ranging from infectious mononucleosis to cancer. In infected cells the virus can adopt several different latency programs, affecting the cells' behaviour. Experimental results indicate that a specific genetic switch between viral latency programs, reprograms human B-cells between proliferative and resting states. Each of these two latency programs makes use of a different viral promoter, Cp and Qp, respectively. The hypothesis tested in this study is that this genetic switch is controlled by both human and viral transcription factors; Oct-2 and EBNA-1. We build a physico-chemical model to investigate quantitatively the dynamical properties of the promoter regulation and experimentally examine protein level variations between the two latency programs.
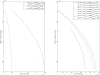
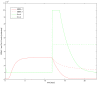
Results: Our experimental results display significant differences in EBNA-1 and Oct-2 levels between resting and proliferating programs. With the model we identify two stable latency programs, corresponding to a resting and proliferating cell. The two programs differ in robustness and transcriptional activity. The proliferating state is markedly more stable, with a very high transcriptional activity from its viral promoter. We predict the promoter activities to be mutually exclusive in the two different programs, and our relative promoter activities correlate well with experimental data. Transitions between programs can be induced, by affecting the protein levels of our transcription factors. Simulated time scales are in line with experimental results.
Conclusion: We show that fundamental properties of the Epstein-Barr virus involvement in latent infection, with implications for tumor biology, can be modelled and understood mathematically. We conclude that EBNA-1 and Oct-2 regulation of Cp and Qp is sufficient to establish mutually exclusive expression patterns. Moreover, the modelled genetic control predict both mono- and bistable behavior and a considerable difference in transition dynamics, based on program stability and promoter activities. Both these phenomena we hope can be further investigated experimentally, to increase the understanding of this important switch. Our results also stress the importance of the little known regulation of human transcription factor Oct-2.
Figures




References
-
- Almqvist J. Epstein-Barr virus nuclear antigen 1, Oct & Groucho/TLE in control of promoter regulation. PhD thesis, Karolinska Institutet. 2005.
-
- Schaefer BC, Woisetschlager M, Strominger JL, Speck SH. Exclusive expression of Epstein-Barr virus nuclear antigen 1 in Burkitts lymphoma arises from a third promoter, distinct from the promoters used in latently infected lymphocytes. Proc Natl Acad Sci. 1991;88:6550–6554. doi: 10.1073/pnas.88.15.6550. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

