Brain mast cell relationship to neurovasculature during development
- PMID: 17764664
- PMCID: PMC2049068
- DOI: 10.1016/j.brainres.2007.07.034
Brain mast cell relationship to neurovasculature during development
Erratum in
- Brain Res. 2008 Jan 23;1190:227
Abstract
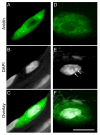
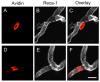
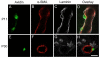
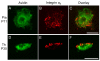
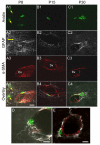
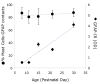
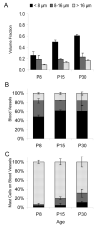
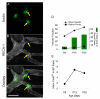
Mast cells, derived from the hematopoietic stem cell, are present in the brain from birth. During development, mast cells occur in two locations, namely the pia and the brain parenchyma. The current hypothesis regarding their origin states that brain mast cells (or their precursors) enter the pia and access the thalamus by traveling along the abluminal wall of penetrating blood vessels. The population in the pia reaches a maximum at postnatal (PN) day 11, and declines rapidly thereafter. Chromatin fragmentation suggests that this cell loss is due to apoptosis. In contrast, the thalamic population expands from PN8 to reach adult levels at PN30. Stereological analysis demonstrates that mast cells home to blood vessels. More than 96% of mast cells are inside the blood-brain barrier, with ~90% contacting the blood vessel wall or its extracellular matrix. Mast cells express alpha4 integrins -- a potential mechanism for adhesion to the vascular wall. Despite the steady increase in the volume of microvasculature, at all ages studied, mast cells are preferentially located on large diameter vessels (>16 microm; possibly arteries), and contact only those maturing blood vessels that are ensheathed by astroglial processes. Mast cells not only home to large vessels but also maintain a preferential position at branch points, sites of vessel growth. This observation presents the possibility that mast cells participate in and/or regulate vasculature growth or differentiation. The biochemical and molecular signals that induce mast cell homing in the CNS is an area of active investigation.
Figures

Similar articles
-
Migration of mast cells in the developing rat brain.Brain Res Dev Brain Res. 1990 Nov 1;56(2):151-9. doi: 10.1016/0165-3806(90)90077-c. Brain Res Dev Brain Res. 1990. PMID: 2261679
-
Mast cells in the neonate musk shrew brain: implications for neuroendocrine immune interactions.Brain Res Dev Brain Res. 1998 Nov 1;111(1):129-36. doi: 10.1016/s0165-3806(98)00121-7. Brain Res Dev Brain Res. 1998. PMID: 9804923
-
Developmental changes of mast cell populations in the cerebral meninges of the rat.J Anat. 2007 Oct;211(4):556-66. doi: 10.1111/j.1469-7580.2007.00795.x. Epub 2007 Sep 6. J Anat. 2007. PMID: 17822416 Free PMC article.
-
[The regulation of mast cell migration. Part 1: cell adhesion molecules].Postepy Hig Med Dosw (Online). 2007 Sep 28;61:485-92. Postepy Hig Med Dosw (Online). 2007. PMID: 17909516 Review. Polish.
-
Blood vessels as a scaffold for neuronal migration.Neurochem Int. 2019 Jun;126:69-73. doi: 10.1016/j.neuint.2019.03.001. Epub 2019 Mar 6. Neurochem Int. 2019. PMID: 30851365 Review.
Cited by
-
Modern Imaging Technologies of Mast Cells for Biology and Medicine (Review).Sovrem Tekhnologii Med. 2021;13(4):93-107. doi: 10.17691/stm2021.13.4.10. Epub 2021 Aug 28. Sovrem Tekhnologii Med. 2021. PMID: 34603768 Free PMC article. Review.
-
Proteoglycans involved in bidirectional communication between mast cells and hippocampal neurons.J Neuroinflammation. 2019 May 20;16(1):107. doi: 10.1186/s12974-019-1504-6. J Neuroinflammation. 2019. PMID: 31109355 Free PMC article.
-
The Role of Mast Cells in Intracerebral Hemorrhage.Neurocrit Care. 2018 Jun;28(3):288-295. doi: 10.1007/s12028-017-0416-5. Neurocrit Care. 2018. PMID: 28620846 Free PMC article. Review.
-
Mast cells and neuroinflammation.Med Sci Monit Basic Res. 2014 Dec 21;20:200-6. doi: 10.12659/MSMBR.893093. Med Sci Monit Basic Res. 2014. PMID: 25529562 Free PMC article. Review.
-
Mast cells are necessary for the hypothermic response to LPS-induced sepsis.Am J Physiol Regul Integr Comp Physiol. 2009 Mar;296(3):R595-602. doi: 10.1152/ajpregu.90888.2008. Epub 2008 Dec 24. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19109365 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

