Generation and characterization of hybridoma antibodies for immunotherapy of tularemia
- PMID: 17764754
- PMCID: PMC2128743
- DOI: 10.1016/j.imlet.2007.07.006
Generation and characterization of hybridoma antibodies for immunotherapy of tularemia
Abstract
Tularemia is caused by the Gram-negative facultative intracellular bacterium Francisella tularensis, which has been classified as a category A select agent-a likely bioweapon. The high virulence of F. tularensis and the threat of engineered antibiotic resistant variants warrant the development of new therapies to combat this disease. We have characterized 14 anti-Francisella hybridoma antibodies derived from mice infected with F. tularensis live vaccine strain (LVS) for potential use as immunotherapy of tularemia. All 14 antibodies cross-reacted with virulent F. tularensis type A clinical isolates, 8 bound to a purified preparation of LVS LPS, and 6 bound to five protein antigens, identified by proteome microarray analysis. An IgG2a antibody, reactive with the LPS preparation, conferred full protection when administered either systemically or intranasally to BALB/c mice post challenge with a lethal dose of intranasal LVS; three other antibodies prolonged survival. These anti-Francisella hybridoma antibodies could be converted to chimeric versions with mouse V regions and human C regions to serve as components of a recombinant polyclonal antibody for clinical testing as immunotherapy of tularemia. The current study is the first to employ proteome microarrays to identify the target antigens of anti-Francisella monoclonal antibodies and the first to demonstrate the systemic and intranasal efficacy of monoclonal antibodies for post-exposure treatment of respiratory tularemia.
Figures

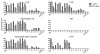

Similar articles
-
Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis.Vaccine. 2007 May 10;25(19):3781-91. doi: 10.1016/j.vaccine.2007.02.014. Epub 2007 Feb 26. Vaccine. 2007. PMID: 17346863 Free PMC article.
-
Immunotherapy of tularemia: characterization of a monoclonal antibody reactive with Francisella tularensis.J Leukoc Biol. 1993 Jan;53(1):112-6. doi: 10.1002/jlb.53.1.112. J Leukoc Biol. 1993. PMID: 8426087
-
Identification of Francisella tularensis outer membrane protein A (FopA) as a protective antigen for tularemia.Vaccine. 2011 Sep 16;29(40):6941-7. doi: 10.1016/j.vaccine.2011.07.075. Epub 2011 Jul 29. Vaccine. 2011. PMID: 21803089 Free PMC article.
-
Live Attenuated Tularemia Vaccines for Protection Against Respiratory Challenge With Virulent F. tularensis subsp. tularensis.Front Cell Infect Microbiol. 2018 May 15;8:154. doi: 10.3389/fcimb.2018.00154. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868510 Free PMC article. Review.
-
Adaptive Immunity to Francisella tularensis and Considerations for Vaccine Development.Front Cell Infect Microbiol. 2018 Apr 6;8:115. doi: 10.3389/fcimb.2018.00115. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29682484 Free PMC article. Review.
Cited by
-
The binding sites of monoclonal antibodies to the non-reducing end of Francisella tularensis O-antigen accommodate mainly the terminal saccharide.Immunology. 2013 Nov;140(3):374-89. doi: 10.1111/imm.12150. Immunology. 2013. PMID: 23844703 Free PMC article.
-
Structural analysis of a protective epitope of the Francisella tularensis O-polysaccharide.Biochemistry. 2012 Jul 17;51(28):5684-94. doi: 10.1021/bi201711m. Epub 2012 Jul 2. Biochemistry. 2012. PMID: 22747335 Free PMC article.
-
Antibodies to both terminal and internal B-cell epitopes of Francisella tularensis O-polysaccharide produced by patients with tularemia.Clin Vaccine Immunol. 2014 Feb;21(2):227-33. doi: 10.1128/CVI.00626-13. Epub 2013 Dec 18. Clin Vaccine Immunol. 2014. PMID: 24351753 Free PMC article.
-
New therapeutic approaches for treatment of tularaemia: a review.Front Cell Infect Microbiol. 2014 Mar 28;4:40. doi: 10.3389/fcimb.2014.00040. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24734221 Free PMC article. Review.
-
Francisella tularensis Vaccines Elicit Concurrent Protective T- and B-Cell Immune Responses in BALB/cByJ Mice.PLoS One. 2015 May 14;10(5):e0126570. doi: 10.1371/journal.pone.0126570. eCollection 2015. PLoS One. 2015. PMID: 25973794 Free PMC article.
References
-
- Sjostedt A. Virulence determinants and protective antigens of Francisella tularensis. Curr Opin Microbiol. 2003;6:66–71. - PubMed
-
- Griffin KF, Oyston PC, Titball RW. Francisella tularensis vaccines. FEMS Immunol Med Microbiol. 2007;49:315–323. - PubMed
-
- Larsson P, Oyston PC, Chain P, Chu MC, Duffield M, Fuxelius HH, Garcia E, Halltorp G, Johansson D, Isherwood KE, Karp PD, Larsson E, Liu Y, Michell S, Prior J, Prior R, Malfatti S, Sjostedt A, Svensson K, Thompson N, Vergez L, Wagg JK, Wren BW, Lindler LE, Andersson SG, Forsman M, Titball RW. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005;37:153–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

