Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress
- PMID: 17765261
- PMCID: PMC2745323
- DOI: 10.1016/j.yjmcc.2007.07.058
Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress
Abstract
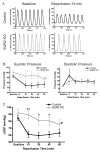
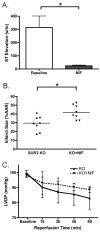
Adenosine triphosphate-sensitive potassium (K(ATP)) channels are thought to mediate the stress response by sensing intracellular ATP concentration. Cardiomyocyte K(ATP) channels are composed of the pore-forming Kir6.2 subunit and the regulatory sulfonylurea receptor 2 (SUR2). We studied the response to acute isoproterenol in SUR2 null mice as a model of acute adrenergic stress and found that the episodic coronary vasospasm observed at baseline in SUR2 null mice was alleviated. Similar results were observed following administration of a nitric oxide donor consistent with a vasodilatory role. Langendorff-perfused hearts were subjected to global ischemia, and hearts from SUR2 null mice exhibited significantly reduced infarct size (54+/-4 versus 30+/-3%) and improved cardiac function compared to control mice. SUR2 null mice have hypertension and develop cardiac hypertrophy. However, despite longstanding hypertension, fibrosis was absent in SUR2 null mice. SUR2 null mice were administered nifedipine to block baseline coronary vasospasm, and hearts from nifedipine-treated SUR2 null mice exhibited increased infarct size compared to untreated SUR2 null mice (42+/-3% versus 54+/-3%). We conclude that conventional sarcolemmal cardiomyocyte K(ATP) channels containing full-length SUR2 are not required for mediating the response to acute cardiovascular stress.
Figures




Similar articles
-
Cardiomyocyte sulfonylurea receptor 2-KATP channel mediates cardioprotection and ST segment elevation.Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H1100-8. doi: 10.1152/ajpheart.00084.2010. Epub 2010 Jul 23. Am J Physiol Heart Circ Physiol. 2010. PMID: 20656890 Free PMC article.
-
Endosomal KATP channels as a reservoir after myocardial ischemia: a role for SUR2 subunits.Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H262-70. doi: 10.1152/ajpheart.00857.2010. Epub 2010 Oct 22. Am J Physiol Heart Circ Physiol. 2011. PMID: 20971764 Free PMC article.
-
Impaired exercise tolerance and skeletal muscle myopathy in sulfonylurea receptor-2 mutant mice.Am J Physiol Regul Integr Comp Physiol. 2009 Oct;297(4):R1144-53. doi: 10.1152/ajpregu.00081.2009. Epub 2009 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19675276 Free PMC article.
-
[Molecular and functional diversity of ATP-sensitive K+ channels: the pathophysiological roles and potential drug targets].Nihon Yakurigaku Zasshi. 2003 Sep;122(3):243-50. doi: 10.1254/fpj.122.243. Nihon Yakurigaku Zasshi. 2003. PMID: 12939542 Review. Japanese.
-
Cardiac sarcolemmal K(ATP) channels: Latest twists in a questing tale!J Mol Cell Cardiol. 2010 Jan;48(1):71-5. doi: 10.1016/j.yjmcc.2009.07.002. Epub 2009 Jul 14. J Mol Cell Cardiol. 2010. PMID: 19607836 Free PMC article. Review.
Cited by
-
ATP-sensitive potassium currents from channels formed by Kir6 and a modified cardiac mitochondrial SUR2 variant.Channels (Austin). 2013 Nov-Dec;7(6):493-502. doi: 10.4161/chan.26181. Epub 2013 Sep 13. Channels (Austin). 2013. PMID: 24037327 Free PMC article.
-
Genetic Discovery of ATP-Sensitive K+ Channels in Cardiovascular Diseases.Circ Arrhythm Electrophysiol. 2019 May;12(5):e007322. doi: 10.1161/CIRCEP.119.007322. Circ Arrhythm Electrophysiol. 2019. PMID: 31030551 Free PMC article. Review.
-
The protective effect of epoxyeicosatrienoic acids on cerebral ischemia/reperfusion injury is associated with PI3K/Akt pathway and ATP-sensitive potassium channels.Neurochem Res. 2015 Jan;40(1):1-14. doi: 10.1007/s11064-014-1456-2. Epub 2014 Nov 4. Neurochem Res. 2015. PMID: 25366463
-
Adenosine Triphosphate-Sensitive Potassium Currents in Heart Disease and Cardioprotection.Card Electrophysiol Clin. 2016 Jun;8(2):323-35. doi: 10.1016/j.ccep.2016.01.005. Epub 2016 Mar 19. Card Electrophysiol Clin. 2016. PMID: 27261824 Free PMC article. Review.
-
Abcc9 is required for the transition to oxidative metabolism in the newborn heart.FASEB J. 2014 Jul;28(7):2804-15. doi: 10.1096/fj.13-244459. Epub 2014 Mar 19. FASEB J. 2014. PMID: 24648545 Free PMC article.
References
-
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. - PubMed
-
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. - PubMed
-
- Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

