Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity
- PMID: 17765279
- PMCID: PMC2610476
- DOI: 10.1016/j.taap.2007.07.016
Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity
Abstract
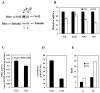
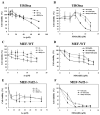
Arsenic is widely spread in our living environment and imposes a big challenge on human health worldwide. Arsenic damages biological systems through multiple mechanisms including the generation of reactive oxygen species. The transcription factor Nrf2 regulates the cellular antioxidant response that protects cells from various insults. In this study, the protective role of Nrf2 in arsenic toxicity was investigated in a human bladder urothelial cell line, UROtsa. Using a UROtsa cell line stably infected with Nrf2-siRNA, we clearly demonstrate that compromised Nrf2 expression sensitized the cells to As(III)- and MMA(III)-induced toxicity. On the other hand, the activation of the Nrf2 pathway by tert-butylhydroquinone (tBHQ) and sulforaphane (SF), the known Nrf2-inducers, rendered UROtsa cells more resistant to As(III) and MMA(III). Furthermore, the wild-type mouse embryo fibroblast (WT-MEF) cells were protected from As(III)- and MMA(III)-induced toxicity following Nrf2 activation by tBHQ or SF, whereas neither tBHQ nor SF conferred protection in the Nrf2(-/-)MEF cells, demonstrating that tBHQ- or SF-mediated protection against As(III)- and MMA(III)-induced toxicity depends on Nrf2 activation. These results, obtained by both loss of function and gain of function analyses, clearly demonstrate the protective role of Nrf2 in arsenic-induced toxicity. The current work lays the groundwork for using Nrf2 activators for therapeutic and dietary interventions against adverse effects of arsenic.
Figures
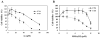
Similar articles
-
Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: enhanced Keap1-Cul3 interaction.Toxicol Appl Pharmacol. 2008 Aug 1;230(3):383-9. doi: 10.1016/j.taap.2008.03.003. Epub 2008 Mar 12. Toxicol Appl Pharmacol. 2008. PMID: 18417180 Free PMC article.
-
Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner.Mol Cell Biol. 2013 Jun;33(12):2436-46. doi: 10.1128/MCB.01748-12. Epub 2013 Apr 15. Mol Cell Biol. 2013. PMID: 23589329 Free PMC article.
-
Nrf2 protects against As(III)-induced damage in mouse liver and bladder.Toxicol Appl Pharmacol. 2009 Oct 1;240(1):8-14. doi: 10.1016/j.taap.2009.06.010. Epub 2009 Jun 16. Toxicol Appl Pharmacol. 2009. PMID: 19538980 Free PMC article.
-
Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway.J Biochem Mol Toxicol. 2013 Feb;27(2):99-105. doi: 10.1002/jbt.21463. Epub 2012 Nov 27. J Biochem Mol Toxicol. 2013. PMID: 23188707 Free PMC article. Review.
-
Immortalized human urothelial cells as a model of arsenic-induced bladder cancer.Toxicology. 2008 Jun 27;248(2-3):67-76. doi: 10.1016/j.tox.2008.03.020. Epub 2008 Mar 30. Toxicology. 2008. PMID: 18456381 Review.
Cited by
-
Protective Role of Nuclear Factor E2-Related Factor 2 against Acute Oxidative Stress-Induced Pancreatic β -Cell Damage.Oxid Med Cell Longev. 2015;2015:639191. doi: 10.1155/2015/639191. Epub 2015 Apr 9. Oxid Med Cell Longev. 2015. PMID: 25949772 Free PMC article.
-
Antioxidants Protect against Arsenic Induced Mitochondrial Cardio-Toxicity.Toxics. 2017 Dec 5;5(4):38. doi: 10.3390/toxics5040038. Toxics. 2017. PMID: 29206204 Free PMC article. Review.
-
Cancer in experimental animals exposed to arsenic and arsenic compounds.Crit Rev Toxicol. 2010 Nov;40(10):912-27. doi: 10.3109/10408444.2010.506641. Crit Rev Toxicol. 2010. PMID: 20812815 Free PMC article. Review.
-
Arsenic exposure and toxicology: a historical perspective.Toxicol Sci. 2011 Oct;123(2):305-32. doi: 10.1093/toxsci/kfr184. Epub 2011 Jul 12. Toxicol Sci. 2011. PMID: 21750349 Free PMC article.
-
Global gene expression changes in human urothelial cells exposed to low-level monomethylarsonous acid.Toxicology. 2012 Jan 27;291(1-3):102-12. doi: 10.1016/j.tox.2011.11.002. Epub 2011 Nov 17. Toxicology. 2012. PMID: 22108045 Free PMC article.
References
-
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. - PubMed
-
- Aposhian HV. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol. 1997;37:397–419. - PubMed
-
- Aposhian HV, Gurzau ES, Le XC, Gurzau A, Healy SM, Lu X, Ma M, Yip L, Zakharyan RA, Maiorino RM, Dart RC, Tircus MG, Gonzalez-Ramirez D, Morgan DL, Avram D, Aposhian MM. Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic. Chem Res Toxicol. 2000;13:693–697. - PubMed
-
- Aposhian HV, Zakharyan RA, Avram MD, Kopplin MJ, Wollenberg ML. Oxidation and detoxification of trivalent arsenic species. Toxicol Appl Pharmacol. 2003;193:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

