Peroxidase activity of de novo heme proteins immobilized on electrodes
- PMID: 17765314
- PMCID: PMC2080791
- DOI: 10.1016/j.jinorgbio.2007.07.024
Peroxidase activity of de novo heme proteins immobilized on electrodes
Abstract
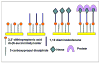
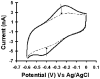
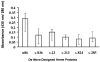
De novo proteins from designed combinatorial libraries were bound to heme terminated gold electrodes. The novel heme proteins were shown to possess peroxidase activity, and this activity was compared to that of horseradish peroxidase and bovine serum albumin when immobilized in a similar fashion. The various designed proteins from the libraries displayed distinctly different levels of peroxidase activity, thereby demonstrating that the sequence and structure of a designed protein can exert a substantial effect on the peroxidase activity of immobilized heme.
Figures


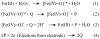
Similar articles
-
Direct electrochemistry of horseradish peroxidase immobilized on electrografted 4-ethynylphenyl film via click chemistry.Anal Chim Acta. 2011 Jul 4;697(1-2):27-31. doi: 10.1016/j.aca.2011.04.035. Epub 2011 Apr 28. Anal Chim Acta. 2011. PMID: 21641415
-
Enzyme biosensor based on the immobilization of HRP on SiO₂/BSA/Au composite nanoparticles.Appl Biochem Biotechnol. 2010 Dec;162(8):2189-96. doi: 10.1007/s12010-010-8993-1. Epub 2010 Jun 9. Appl Biochem Biotechnol. 2010. PMID: 20532672
-
[Comparative study of horseradish peroxidase immobilization on modification of a gold surface using a surface plasmon resonance method].Ukr Biokhim Zh (1999). 2001 Mar-Apr;73(2):44-50. Ukr Biokhim Zh (1999). 2001. PMID: 11642043 Ukrainian.
-
MgFe-layered double hydroxide modified electrodes for direct electron transfer of heme proteins.Biosens Bioelectron. 2012 Oct-Dec;38(1):239-44. doi: 10.1016/j.bios.2012.05.035. Epub 2012 Jun 6. Biosens Bioelectron. 2012. PMID: 22721646
-
Aromatic donor molecule binding sites of haem peroxidases.Biochem Soc Trans. 1995 May;23(2):232-40. doi: 10.1042/bst0230232. Biochem Soc Trans. 1995. PMID: 7672252 Review. No abstract available.
Cited by
-
Design of functional metalloproteins.Nature. 2009 Aug 13;460(7257):855-62. doi: 10.1038/nature08304. Nature. 2009. PMID: 19675646 Free PMC article. Review.
-
Mechanisms and physiological function of daily haemoglobin oxidation rhythms in red blood cells.EMBO J. 2023 Oct 4;42(19):e114164. doi: 10.15252/embj.2023114164. Epub 2023 Aug 9. EMBO J. 2023. PMID: 37554073 Free PMC article.
-
Protein design: toward functional metalloenzymes.Chem Rev. 2014 Apr 9;114(7):3495-578. doi: 10.1021/cr400458x. Epub 2014 Mar 24. Chem Rev. 2014. PMID: 24661096 Free PMC article. Review. No abstract available.
-
Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer.Biochemistry. 2009 Dec 29;48(51):12104-12. doi: 10.1021/bi9011435. Biochemistry. 2009. PMID: 19908820 Free PMC article.
-
Immuno-spin trapping of heme-induced protein radicals: Implications for heme oxygenase-1 induction and heme degradation.Free Radic Biol Med. 2013 Aug;61:265-72. doi: 10.1016/j.freeradbiomed.2013.04.026. Epub 2013 Apr 25. Free Radic Biol Med. 2013. PMID: 23624303 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

