In vitro and in vivo demonstration of risperidone implants in mice
- PMID: 17765477
- PMCID: PMC2561216
- DOI: 10.1016/j.schres.2007.08.003
In vitro and in vivo demonstration of risperidone implants in mice
Abstract
Background: Non-adherence with medication is a critical limitation in current long-term treatment of schizophrenia and a primary factor in poor quality-of-life outcomes. However, few treatments have addressed this shortcoming using an implantable drug delivery approach. The goal of this study was to provide in vitro and in vivo proof of concept for a long-term implantable risperidone delivery system in mice.
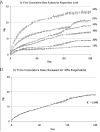
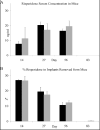
Methods: Implantable formulations of risperidone were created using the biodegradable polymer Poly Lactic co Glycolic Acid (PLGA) combined with various drug loads. Implant bioactivity was tested using in vitro release and stability studies, as well as in vivo pharmacokinetic and behavioral studies in mice.
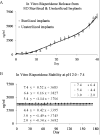
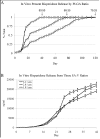
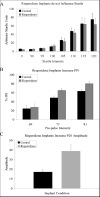
Results: The pattern of risperidone release is influenced by various parameters, including polymer composition and drug load. In vitro measures demonstrate that risperidone is stable in implants under physiological conditions. Behavioral measures demonstrate the bioactivity of risperidone implants delivering 3 mg/kg/day in mice, while pharmacokinetic analyses indicate that reversibility is maintained throughout the delivery interval.
Conclusions: The current report suggests that implantable formulations are a viable approach to providing long-term delivery of antipsychotic medications based on in vivo animal studies and pharmacokinetics. Implantable medications demonstrated here can last two months or longer while maintaining coherence and removability past full release, suggesting a potential paradigm shift in the long-term treatment of schizophrenia.
Figures

References
-
- Barnes TR, Curson DA. Long-term depot antipsychotics. A risk-benefit assessment. Drug Saf. 1994;10:464–479. - PubMed
-
- Bloch YMS, Strupinsky S, Altshuler A, Fennig S, Ratzoni G. Injections of depot antipsychotic medications in patients suffering from schizophrenia: do they hurt? Journal of Clinical Psychiatry. 2001;62:855–859. - PubMed
-
- Boutros N, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Research. 2004;126:203–215. - PubMed
-
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, et al. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. - PubMed
-
- de Oliveira IM-SAdSEPERMdC-e-SEBJ. Risperidone versus haloperidol in the treatment of schizophrenia: a meta-analysis comparing their efficacy and safety. J Clin Pharm Ther. 1996;21:349–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

