GEF what? Dock180 and related proteins help Rac to polarize cells in new ways
- PMID: 17765544
- PMCID: PMC2887429
- DOI: 10.1016/j.tcb.2007.05.001
GEF what? Dock180 and related proteins help Rac to polarize cells in new ways
Abstract
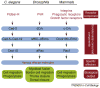
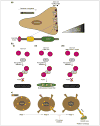
Rho GTPase activation, which is mediated by guanine nucleotide exchange factors (GEFs), is tightly regulated in time and space. Although Rho GTPases have a significant role in many biological events, they are best known for their ability to restructure the actin cytoskeleton profoundly through the activation of specific downstream effectors. Two distinct families of GEFs for Rho GTPases have been reported so far, based on the features of their catalytic domains: firstly, the classical GEFs, which contain a Dbl homology-pleckstrin homology domain module with GEF activity, and secondly, the Dock180-related GEFs, which contain a Dock homology region-2 domain that catalyzes guanine nucleotide exchange on Rho GTPases. Recent exciting data suggest key roles for the DHR-2 domain-containing GEFs in a wide variety of fundamentally important biological functions, including cell migration, phagocytosis of apoptotic cells, myoblast fusion and neuronal polarization.
Figures





Similar articles
-
Dock180-ELMO cooperation in Rac activation.Methods Enzymol. 2006;406:388-402. doi: 10.1016/S0076-6879(06)06028-9. Methods Enzymol. 2006. PMID: 16472672
-
Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs).J Biol Chem. 2010 Apr 23;285(17):13211-22. doi: 10.1074/jbc.M110.102517. Epub 2010 Feb 18. J Biol Chem. 2010. PMID: 20167601 Free PMC article.
-
A minimal Rac activation domain in the unconventional guanine nucleotide exchange factor Dock180.Biochemistry. 2011 Feb 15;50(6):1070-80. doi: 10.1021/bi100971y. Epub 2011 Jan 20. Biochemistry. 2011. PMID: 21033699 Free PMC article.
-
CZH proteins: a new family of Rho-GEFs.J Cell Sci. 2005 Nov 1;118(Pt 21):4937-46. doi: 10.1242/jcs.02671. J Cell Sci. 2005. PMID: 16254241 Review.
-
Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement.Cells. 2022 Nov 11;11(22):3565. doi: 10.3390/cells11223565. Cells. 2022. PMID: 36428994 Free PMC article. Review.
Cited by
-
The SH3 regulatory domain of the hematopoietic cell kinase Hck binds ELMO via its polyproline motif.FEBS Open Bio. 2015 Feb 4;5:99-106. doi: 10.1016/j.fob.2015.01.009. eCollection 2015. FEBS Open Bio. 2015. PMID: 25737835 Free PMC article.
-
Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes TH17 cell differentiation.J Allergy Clin Immunol. 2016 Nov;138(5):1384-1394.e2. doi: 10.1016/j.jaci.2016.04.023. Epub 2016 May 24. J Allergy Clin Immunol. 2016. PMID: 27350570 Free PMC article.
-
Regulatory role of guanine nucleotide exchange factor (GEF) Dock180 phosphorylation on Tyr/Ser in mediation of gastric mucosal Rac1 activation in response to Helicobacter pylori and ghrelin.Inflammopharmacology. 2015 Jun;23(2-3):111-8. doi: 10.1007/s10787-015-0235-2. Epub 2015 May 10. Inflammopharmacology. 2015. PMID: 25957600
-
Independent recognition of Staphylococcus aureus by two receptors for phagocytosis in Drosophila.J Biol Chem. 2012 Jun 22;287(26):21663-72. doi: 10.1074/jbc.M111.333807. Epub 2012 Apr 30. J Biol Chem. 2012. PMID: 22547074 Free PMC article.
-
Structural basis for mutual relief of the Rac guanine nucleotide exchange factor DOCK2 and its partner ELMO1 from their autoinhibited forms.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3305-10. doi: 10.1073/pnas.1113512109. Epub 2012 Feb 13. Proc Natl Acad Sci U S A. 2012. PMID: 22331897 Free PMC article.
References
-
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. - PubMed
-
- Rossman KL, et al. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. - PubMed
-
- Meller N, et al. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–4946. - PubMed
-
- Brugnera E, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–582. - PubMed
-
- Meller N, et al. The novel Cdc42 guanine nucleotide exchange factor, zizimin1, dimerizes via the Cdc42-binding CZH2 domain. J Biol Chem. 2004;279:37470–37476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

