A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication
- PMID: 17765705
- PMCID: PMC7112299
- DOI: 10.1016/S0065-3527(07)70004-0
A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication
Abstract
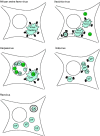
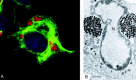
Virus replication can cause extensive rearrangement of host cell cytoskeletal and membrane compartments leading to the "cytopathic effect" that has been the hallmark of virus infection in tissue culture for many years. Recent studies are beginning to redefine these signs of viral infection in terms of specific effects of viruses on cellular processes. In this chapter, these concepts have been illustrated by describing the replication sites produced by many different viruses. In many cases, the cellular rearrangements caused during virus infection lead to the construction of sophisticated platforms in the cell that concentrate replicase proteins, virus genomes, and host proteins required for replication, and thereby increase the efficiency of replication. Interestingly, these same structures, called virus factories, virus inclusions, or virosomes, can recruit host components that are associated with cellular defences against infection and cell stress. It is possible that cellular defence pathways can be subverted by viruses to generate sites of replication. The recruitment of cellular membranes and cytoskeleton to generate virus replication sites can also benefit viruses in other ways. Disruption of cellular membranes can, for example, slow the transport of immunomodulatory proteins to the surface of infected cells and protect against innate and acquired immune responses, and rearrangements to cytoskeleton can facilitate virus release.
Figures







References
-
- Adair R., Douglas E.R., Maclean J.B., Graham S.Y., Aitken J.D., Jamieson F.E., Dargan D.J. The products of human cytomegalovirus genes UL23, UL24, UL43 and US22 are tegument components. J. Gen. Virol. 2002;83:1315–1324. - PubMed
-
- Alcamí A., Angulo A., Viñuela E. Mapping and sequence of the gene encoding the African swine fever virion protein of Mr 11500. J. Gen. Virol. 1993;74:2317–2324. - PubMed
-
- Aldabe R., Barco A., Carrasco L. Membrane permeabilization by poliovirus proteins 2B and 2BC. J. Biol. Chem. 1996;271:23134–23137. - PubMed
Further Reading
-
- Raoult D., La Scola B., Birtles R. The discovery and characterization of mimivirus, the largest known virus and putative pneumonia agent. Clin. Infect. Dis. 1986;45:95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

