The CA3 "backprojection" to the dentate gyrus
- PMID: 17765742
- PMCID: PMC1986638
- DOI: 10.1016/S0079-6123(07)63034-9
The CA3 "backprojection" to the dentate gyrus
Abstract
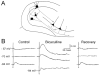
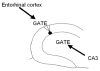
The hippocampus is typically described in the context of the trisynaptic circuit, a pathway that relays information from the perforant path to the dentate gyrus, dentate to area CA3, and CA3 to area CA1. Associated with this concept is the assumption that most hippocampal information processing occurs along the trisynaptic circuit. However, the entorhinal cortex may not be the only major extrinsic input to consider, and the trisynaptic circuit may not be the only way information is processed in hippocampus. Area CA3 receives input from a variety of sources, and may be as much of an "entry point" to hippocampus as the dentate gyrus. The axon of CA3 pyramidal cells targets diverse cell types, and has commissural projections, which together make it able to send information to much more of the hippocampus than granule cells. Therefore, CA3 pyramidal cells seem better designed to spread information through hippocampus than the granule cells. From this perspective, CA3 may be a point of entry that receives information which needs to be "broadcasted," whereas the dentate gyrus may be a point of entry that receives information with more selective needs for hippocampal processing. One aspect of the argument that CA3 pyramidal cells have a widespread projection is based on a part of its axonal arbor that has received relatively little attention, the collaterals that project in the opposite direction to the trisynaptic circuit, "back" to the dentate gyrus. The evidence for this "backprojection" to the dentate gyrus is strong, particularly in area CA3c, the region closest to the dentate gyrus, and in temporal hippocampus. The influence on granule cells is indirect, through hilar mossy cells and GABAergic neurons of the dentate gyrus, and appears to include direct projections in the case of CA3c pyramidal cells of ventral hippocampus. Physiological studies suggest that normally area CA3 does not have a robust excitatory influence on granule cells, but serves instead to inhibit it by activating dentate gyrus GABAergic neurons. Thus, GABAergic inhibition normally controls the backprojection to dentate granule cells, analogous to the way GABAergic inhibition appears to control the perforant path input to granule cells. From this perspective, the dentate gyrus has two robust glutamatergic inputs, entorhinal cortex and CA3, and two "gates," or inhibitory filters that reduce the efficacy of both inputs, keeping granule cells relatively quiescent. When GABAergic inhibition is reduced experimentally, or under pathological conditions, CA3 pyramidal cells activate granule cells reliably, and do so primarily by disynaptic excitation that is mediated by mossy cells. We suggest that the backprojection has important functions normally that are dynamically regulated by nonprincipal cells of the dentate gyrus. Slightly reduced GABAergic input would lead to increased polysynaptic associative processing between CA3 and the dentate gyrus. Under pathological conditions associated with loss of GABAergic interneurons, the backprojection may support reverberatory excitatory activity between CA3, mossy cells, and granule cells, possibly enhanced by mossy fiber sprouting. In this case, the backprojection could be important to seizure activity originating in hippocampus, and help explain the seizure susceptibility of ventral hippocampus.
Figures
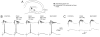
Similar articles
-
Conditions required for polysynaptic excitation of dentate granule cells by area CA3 pyramidal cells in rat hippocampal slices.Neuroscience. 1996 Jun;72(3):655-68. doi: 10.1016/0306-4522(95)00569-2. Neuroscience. 1996. PMID: 9157312 Free PMC article.
-
EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar "mossy" cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices.J Neurosci. 1994 Oct;14(10):6041-57. doi: 10.1523/JNEUROSCI.14-10-06041.1994. J Neurosci. 1994. PMID: 7931561 Free PMC article.
-
Local and Long-Range Circuit Connections to Hilar Mossy Cells in the Dentate Gyrus.eNeuro. 2017 Apr 19;4(2):ENEURO.0097-17.2017. doi: 10.1523/ENEURO.0097-17.2017. eCollection 2017 Mar-Apr. eNeuro. 2017. PMID: 28451637 Free PMC article.
-
Mossy fiber synaptic transmission: communication from the dentate gyrus to area CA3.Prog Brain Res. 2007;163:109-32. doi: 10.1016/S0079-6123(07)63006-4. Prog Brain Res. 2007. PMID: 17765714 Review.
-
Divergence of hippocampal mossy fibers.Synapse. 1994 Feb;16(2):148-60. doi: 10.1002/syn.890160208. Synapse. 1994. PMID: 8197576 Review.
Cited by
-
Noncanonical connections between the subiculum and hippocampal CA1.J Comp Neurol. 2016 Dec 1;524(17):3666-3673. doi: 10.1002/cne.24024. Epub 2016 May 6. J Comp Neurol. 2016. PMID: 27150503 Free PMC article. Review.
-
JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche.Mol Psychiatry. 2018 Feb;23(2):362-374. doi: 10.1038/mp.2016.203. Epub 2016 Nov 15. Mol Psychiatry. 2018. PMID: 27843149 Free PMC article.
-
Functional differentiation in the transverse plane of the hippocampus: An update on activity segregation within the DG and CA3 subfields.Brain Res Bull. 2021 Jun;171:35-43. doi: 10.1016/j.brainresbull.2021.03.003. Epub 2021 Mar 13. Brain Res Bull. 2021. PMID: 33727088 Free PMC article. Review.
-
Pattern separation in the dentate gyrus: a role for the CA3 backprojection.Hippocampus. 2011 Nov;21(11):1190-215. doi: 10.1002/hipo.20828. Epub 2010 Aug 3. Hippocampus. 2011. PMID: 20683841 Free PMC article.
-
Adult hippocampal neurogenesis and its role in cognition.Wiley Interdiscip Rev Cogn Sci. 2014 Sep;5(5):573-587. doi: 10.1002/wcs.1304. Epub 2014 Aug 12. Wiley Interdiscip Rev Cogn Sci. 2014. PMID: 26308746 Free PMC article.
References
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Bilkey DK, Schwartzkroin PA. Variation in electrophysiology and morphology of hippocampal CA3 pyramidal cells. Brain Res. 1990;514:77–83. - PubMed
-
- Borck C, Jefferys JG. Seizure-like events in disinhibited ventral slices of adult rat hippocampus. J Neurophysiol. 1999;82:2130–2142. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

