New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer
- PMID: 17765940
- PMCID: PMC2740485
- DOI: 10.1016/j.steroids.2007.07.009
New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer
Abstract
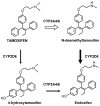
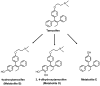
The metabolism of tamoxifen is being redefined in the light of several important pharmacological observations. Recent studies have identified 4-hydroxy N-desmethyltamoxifen (endoxifen) as an important metabolite of tamoxifen necessary for antitumor actions. The metabolite is formed through the enzymatic product of CYP2D6 which also interacts with specific selective serotonin reuptake inhibitors (SSRIs) used to prevent the hot flashes observed in up to 45% of patients taking tamoxifen. Additionally, the finding that enzyme variants of CYP2D6 do not promote the metabolism of tamoxifen to endoxifen means that significant numbers of women might not receive optimal benefit from tamoxifen treatment. Clearly these are particularly important issues not only for breast cancer treatment but also for selecting premenopausal women, at high risk for breast cancer, as candidates for chemoprevention using tamoxifen.
Figures
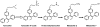

Similar articles
-
Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine.J Natl Cancer Inst. 2003 Dec 3;95(23):1758-64. doi: 10.1093/jnci/djg108. J Natl Cancer Inst. 2003. PMID: 14652237 Clinical Trial.
-
Understanding resistance to tamoxifen in hormone receptor-positive breast cancer.Clin Chem. 2009 Aug;55(8):1453-5. doi: 10.1373/clinchem.2009.125377. Epub 2009 Jun 18. Clin Chem. 2009. PMID: 19541862 No abstract available.
-
CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment.J Natl Cancer Inst. 2005 Jan 5;97(1):30-9. doi: 10.1093/jnci/dji005. J Natl Cancer Inst. 2005. PMID: 15632378
-
Should CYP2D6 inhibitors be administered in conjunction with tamoxifen?Expert Rev Anticancer Ther. 2011 Feb;11(2):185-93. doi: 10.1586/era.10.228. Expert Rev Anticancer Ther. 2011. PMID: 21342038 Review.
-
Targeting of tamoxifen to enhance antitumour action for the treatment and prevention of breast cancer: the 'personalised' approach?Eur J Cancer. 2009 Sep;45(13):2274-83. doi: 10.1016/j.ejca.2009.05.032. Epub 2009 Jul 9. Eur J Cancer. 2009. PMID: 19592233 Review.
Cited by
-
Generating a Precision Endoxifen Prediction Algorithm to Advance Personalized Tamoxifen Treatment in Patients with Breast Cancer.J Pers Med. 2021 Mar 13;11(3):201. doi: 10.3390/jpm11030201. J Pers Med. 2021. PMID: 33805613 Free PMC article. Review.
-
BreastDefend enhances effect of tamoxifen in estrogen receptor-positive human breast cancer in vitro and in vivo.BMC Complement Altern Med. 2017 Feb 16;17(1):115. doi: 10.1186/s12906-017-1621-7. BMC Complement Altern Med. 2017. PMID: 28209156 Free PMC article.
-
ESR1 promoter hypermethylation does not predict atypia in RPFNA nor persistent atypia after 12 months tamoxifen chemoprevention.Cancer Epidemiol Biomarkers Prev. 2008 Aug;17(8):1884-90. doi: 10.1158/1055-9965.EPI-07-2696. Cancer Epidemiol Biomarkers Prev. 2008. PMID: 18708376 Free PMC article.
-
Precision Oncology Medicine: The Clinical Relevance of Patient-Specific Biomarkers Used to Optimize Cancer Treatment.J Clin Pharmacol. 2016 Dec;56(12):1484-1499. doi: 10.1002/jcph.765. Epub 2016 Jun 17. J Clin Pharmacol. 2016. PMID: 27197880 Free PMC article. Review.
-
Mammographic Density as a Biosensor of Tamoxifen Effectiveness in Adjuvant Endocrine Treatment of Breast Cancer: Opportunities and Implications.J Clin Oncol. 2016 Jun 20;34(18):2093-7. doi: 10.1200/JCO.2015.64.4492. Epub 2016 Mar 28. J Clin Oncol. 2016. PMID: 27022110 Free PMC article. No abstract available.
References
-
- Snyder KR, Sparano N, Malinowski JM. Raloxifene hydrochloride. Am J Health Syst Pharm. 2000;57(18):1669–1675. quiz 1676–1678. - PubMed
-
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nature Reviews Drug Discovery. 2003;2:205–213. - PubMed
-
- Harper MJ, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966;212(57):87. - PubMed
-
- Harper MJ, Walpole AL. Mode of action of I.C.I. 46,474 in preventing implantation in rats. J Endocrinol. 1967;37(1):83–92. - PubMed
-
- Harper MJ, Walpole AL. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967;13:101–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

