Analysis of nociception, sex and peripheral nerve innervation in the TMEV animal model of multiple sclerosis
- PMID: 17766043
- PMCID: PMC2673489
- DOI: 10.1016/j.pain.2007.07.007
Analysis of nociception, sex and peripheral nerve innervation in the TMEV animal model of multiple sclerosis
Abstract
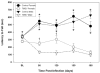
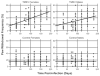
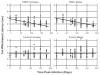
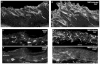
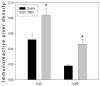
Although pain was previously not considered an important element of multiple sclerosis (MS), recent evidence indicates that over 50% of MS patients suffer from chronic pain. In the present study, we utilized the Theiler's murine encephalomyelitis virus (TMEV) model of MS to examine whether changes in nociception occur during disease progression and to investigate whether sex influences the development of nociception or disease-associated neurological symptoms. Using the rotarod assay, TMEV infected male mice displayed increased neurological deficits when compared to TMEV infected female mice, which mimics what is observed in human MS. While both male and female TMEV infected mice exhibited thermal hyperalgesia and mechanical allodynia, female mice developed mechanical allodynia at a faster rate and displayed significantly more mechanical allodynia than male mice. Since neuropathic symptoms have been described in MS patients, we quantified sensory nerve fibers in the epidermis of TMEV-infected and non-infected mice to determine if there were alterations in epidermal nerve density. There was a significantly higher density of PGP9.5 and CGRP-immunoreactive axons in the epidermis of TMEV-infected mice versus controls. Collectively these results indicate that the TMEV model is well suited to study the mechanisms of MS-induced nociception and suggest that alterations in peripheral nerve innervation may contribute to MS pain.
Figures

Similar articles
-
Decreased spinal cord opioid receptor mRNA expression and antinociception in a Theiler's murine encephalomyelitis virus model of multiple sclerosis.Brain Res. 2008 Jan 29;1191:180-91. doi: 10.1016/j.brainres.2007.11.034. Epub 2007 Nov 28. Brain Res. 2008. PMID: 18096140 Free PMC article.
-
More severe neurologic deficits in SJL/J male than female mice following Theiler's virus-induced CNS demyelination.Exp Neurol. 2003 Mar;180(1):14-24. doi: 10.1016/s0014-4886(02)00054-7. Exp Neurol. 2003. PMID: 12668145
-
Longitudinal quantitative assessment of TMEV-IDD-induced MS phenotypes in two inbred mouse strains using automated video tracking technology.Exp Neurol. 2024 Sep;379:114851. doi: 10.1016/j.expneurol.2024.114851. Epub 2024 Jun 13. Exp Neurol. 2024. PMID: 38876197
-
[Theiler's virus encephalomyelitis infection as a model for multiple sclerosis: cytokines and pathogenic mechanisms].Rev Neurol. 2002 Nov 16-30;35(10):973-8. Rev Neurol. 2002. PMID: 12436402 Review. Spanish.
-
Theiler's virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes.Virus Res. 2005 Aug;111(2):214-23. doi: 10.1016/j.virusres.2005.04.010. Virus Res. 2005. PMID: 15893838 Review.
Cited by
-
Ovalbumin-specific CD4+ and CD8+ T cells contribute to different susceptibility for Theiler's murine encephalomyelitis virus persistence.Front Immunol. 2023 May 24;14:1194842. doi: 10.3389/fimmu.2023.1194842. eCollection 2023. Front Immunol. 2023. PMID: 37292191 Free PMC article.
-
Sex differences in a mouse model of multiple sclerosis: neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus.Biol Sex Differ. 2014 Feb 28;5(1):4. doi: 10.1186/2042-6410-5-4. Biol Sex Differ. 2014. PMID: 24581045 Free PMC article.
-
Social disruption alters pain and cognition in an animal model of multiple sclerosis.J Neuroimmunol. 2015 Nov 15;288:56-68. doi: 10.1016/j.jneuroim.2015.09.005. Epub 2015 Sep 12. J Neuroimmunol. 2015. PMID: 26531695 Free PMC article.
-
Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy.Brain Behav Immun. 2017 Jan;59:49-54. doi: 10.1016/j.bbi.2016.05.012. Epub 2016 May 14. Brain Behav Immun. 2017. PMID: 27189037 Free PMC article.
-
Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside.Neurotherapeutics. 2009 Oct;6(4):713-37. doi: 10.1016/j.nurt.2009.08.002. Neurotherapeutics. 2009. PMID: 19789075 Free PMC article. Review.
References
-
- Aicher SA, Silverman MB, Winkler CW, Bebo BF., Jr Hyperalgesia in an animal model of multiple sclerosis. Pain. 2004;110:560–570. - PubMed
-
- Alley J, Khasabov S, Simone D, Beitz A, Rodriguez M, Njenga MK. More severe neurologic deficits in SJL/J male than female mice following Theiler’s virus-induced CNS demyelination. Exp Neurol. 2003;180:14–24. - PubMed
-
- Al-Smadi J, Warke K, Wilson I, Cramp AF, Noble G, Walsh DM, Lowe-Strong AS. A pilot investigation of the hypoalgesic effects of transcutaneous electrical nerve stimulation upon low back pain in people with multiple sclerosis. Clin Rehabil. 2003;17:742–749. - PubMed
-
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, Maxner CE, Murray TJ. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain. 1994;58:89–93. - PubMed
-
- Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technology Assessment. 2003;7:1–124. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

