Rings, bracelets, sleeves, and chevrons: new structures of kinetochore proteins
- PMID: 17766118
- PMCID: PMC2908952
- DOI: 10.1016/j.tcb.2007.08.001
Rings, bracelets, sleeves, and chevrons: new structures of kinetochore proteins
Abstract
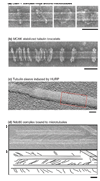
Electron microscopy has recently revealed striking structural orderliness in kinetochore proteins and protein complexes that associate with microtubules. In addition to their astonishing appearance and intrinsic beauty, the structures are functionally informative. The Dam1 and Ndc80 complexes bind to the microtubule lattice as rings and chevrons, respectively. These structures give insight into how the kinetochore couples to dynamic microtubules, a process crucial to the accurate segregation of chromosomes. HURP and kinesin-13 arrange tubulin into sleeves and bracelets surrounding the microtubule lattice. These structures might reflect the ability of these proteins to modulate microtubule dynamics by interacting with specialized tubulin configurations. In this review, we compare and contrast the structure of these proteins and their interactions with microtubules to illustrate how they attach to and modulate the dynamics of microtubules.
Figures

Similar articles
-
Molecular requirements for the formation of a kinetochore-microtubule interface by Dam1 and Ndc80 complexes.J Cell Biol. 2013 Jan 7;200(1):21-30. doi: 10.1083/jcb.201210091. Epub 2012 Dec 31. J Cell Biol. 2013. PMID: 23277429 Free PMC article.
-
Structure of the DASH/Dam1 complex shows its role at the yeast kinetochore-microtubule interface.Science. 2018 May 4;360(6388):552-558. doi: 10.1126/science.aar6436. Science. 2018. PMID: 29724956 Free PMC article.
-
Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex.Mol Cell. 2005 Jan 21;17(2):277-90. doi: 10.1016/j.molcel.2004.12.019. Mol Cell. 2005. PMID: 15664196
-
Kinetochore-microtubule interactions: the means to the end.Curr Opin Cell Biol. 2008 Feb;20(1):53-63. doi: 10.1016/j.ceb.2007.11.005. Epub 2008 Jan 7. Curr Opin Cell Biol. 2008. PMID: 18182282 Free PMC article. Review.
-
Structures and functions of yeast kinetochore complexes.Annu Rev Biochem. 2007;76:563-91. doi: 10.1146/annurev.biochem.76.052705.160607. Annu Rev Biochem. 2007. PMID: 17362199 Review.
Cited by
-
Structural basis of microtubule depolymerization by the kinesin-like activity of HIV-1 Rev.Structure. 2023 Oct 5;31(10):1233-1246.e5. doi: 10.1016/j.str.2023.07.009. Epub 2023 Aug 11. Structure. 2023. PMID: 37572662 Free PMC article.
-
Tubulin depolymerization may be an ancient biological motor.J Cell Sci. 2010 Oct 15;123(Pt 20):3425-34. doi: 10.1242/jcs.067611. J Cell Sci. 2010. PMID: 20930138 Free PMC article. Review.
-
Sister kinetochore recapture in fission yeast occurs by two distinct mechanisms, both requiring Dam1 and Klp2.Mol Biol Cell. 2008 Apr;19(4):1646-62. doi: 10.1091/mbc.e07-09-0910. Epub 2008 Feb 6. Mol Biol Cell. 2008. PMID: 18256284 Free PMC article.
-
Production and initial characterization of Dad1p, a component of the Dam1-DASH kinetochore complex.PLoS One. 2008;3(12):e3888. doi: 10.1371/journal.pone.0003888. Epub 2008 Dec 9. PLoS One. 2008. PMID: 19065263 Free PMC article.
-
Structure of the kinesin13-microtubule ring complex.Structure. 2008 Nov 12;16(11):1732-9. doi: 10.1016/j.str.2008.08.017. Structure. 2008. PMID: 19000825 Free PMC article.
References
-
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K. Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol. 2004 Nov 9;14(21):1962–1967. - PubMed
-
- Koshland DE, Mitchison TJ, Kirschner MW. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. - PubMed
-
- Moores CA, Cooper J, Wagenbach M, Ovechkina Y, Wordeman L, Milligan RA. The role of the kinesin-13 neck in microtubule depolymerization. Cell Cycle. 2006 Aug;5(16):1812–1815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

