Effects of sustained length-dependent activation on in situ cross-bridge dynamics in rat hearts
- PMID: 17766361
- PMCID: PMC2098739
- DOI: 10.1529/biophysj.107.111740
Effects of sustained length-dependent activation on in situ cross-bridge dynamics in rat hearts
Abstract
The cellular basis of the length-dependent increases in contractile force in the beating heart has remained unclear. Our aim was to investigate whether length-dependent mediated increases in contractile force are correlated with myosin head proximity to actin filaments, and presumably the number of cross-bridges activated during a contraction. We therefore employed x-ray diffraction analyses of beat-to-beat contractions in spontaneously beating rat hearts under open-chest conditions simultaneous with recordings of left ventricle (LV) pressure-volume. Regional x-ray diffraction patterns were recorded from the anterior LV free wall under steady-state contractions and during acute volume loading (intravenous lactate Ringers infusion at 60 ml/h, <5 min duration) to determine the change in intensity ratio (I(1,0)/I(1,1)) and myosin interfilament spacing (d(1,0)). We found no significant change in end-diastolic (ED) intensity ratio, indicating that the proportion of myosin heads in proximity to actin was unchanged by fiber stretching. Intensity ratio decreased significantly more during the isovolumetric contraction phase during volume loading than under baseline contractions. A significant systolic increase in myosin head proximity to actin filaments correlated with the maximum rate of pressure increase. Hence, a reduction in interfilament spacing at end-diastole ( approximately 0.5 nm) during stretch increased the proportion of cross-bridges activated. Furthermore, our recordings suggest that d(1,0) expansion was inversely related to LV volume but was restricted during contraction and sarcomere shortening to values smaller than the maximum during isovolumetric relaxation. Since ventricular volume, and presumably sarcomere length, was found to be directly related to interfilament spacing, these findings support a role for interfilament spacing in modulating cross-bridge formation and force developed before shortening.
Figures
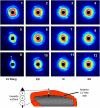
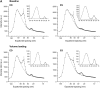


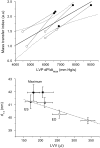
Comment in
-
Mechanisms of the Frank-Starling law of the heart: the beat goes on.Biophys J. 2007 Dec 15;93(12):4095-6. doi: 10.1529/biophysj.107.117200. Epub 2007 Aug 31. Biophys J. 2007. PMID: 17766360 Free PMC article. Review. No abstract available.
References
-
- Lew, W. Y. 1988. Time-dependent increase in left ventricular contractility following acute volume loading in the dog. Circ. Res. 63:635–647. - PubMed
-
- Cingolani, H. E., N. G. Perez, and M. C. Camilion de Hurtado. 2001. An autocrine/paracrine mechanism triggered by myocardial stretch induces changes in contractility. News Physiol. Sci. 16:88–91. - PubMed
-
- Fuchs, F., and S. H. Smith. 2001. Calcium, cross-bridges, and the Frank-Starling relationship. News Physiol. Sci. 16:5–10. - PubMed
-
- Ross, J., Jr., T. Miura, M. Kambayashi, G. P. Eising, and K.-H. Ryu. 1995. Adrenergic control of the force-frequency relation. Circulation. 92:2327–2332. - PubMed
-
- Kentish, J. C., H. E. ter Keurs, L. Ricciardi, J. J. Bucx, and M. I. Noble. 1986. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ. Res. 58:755–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

