Conformational changes induced by a single amino acid substitution in the trans-membrane domain of Vpu: implications for HIV-1 susceptibility to channel blocking drugs
- PMID: 17766368
- PMCID: PMC2204142
- DOI: 10.1110/ps.073041107
Conformational changes induced by a single amino acid substitution in the trans-membrane domain of Vpu: implications for HIV-1 susceptibility to channel blocking drugs
Abstract
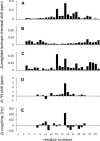
The channel-forming trans-membrane domain of Vpu (Vpu TM) from HIV-1 is known to enhance virion release from the infected cells and is a potential target for ion-channel blockers. The substitution of alanine at position 18 by a histidine (A18H) has been shown to render HIV-1 infections susceptible to rimantadine, a channel blocker of M2 protein from the influenza virus. In order to describe the influence of the mutation on the structure and rimantadine susceptibility of Vpu, we determined the structure of A18H Vpu TM, and compared it to those of wild-type Vpu TM and M2 TM. Both isotropic and orientationally dependent NMR frequencies of the backbone amide resonance of His18 were perturbed by rimantadine, and those of Ile15 and Trp22 were also affected, suggesting that His18 is the key residue for rimantadine binding and that residues located on the same face of the TM helix are also involved. A18H Vpu TM has an ideal, straight alpha-helix spanning residues 6-27 with an average tilt angle of 41 degrees in C14 phospholipid bicelles, indicating that the tilt angle is increased by 11 degrees compared to that of wild-type Vpu TM. The longer helix formed by the A18H mutation has a larger tilt angle to compensate for the hydrophobic mismatch with the length of the phospholipids in the bilayer. These results demonstrate that the local change of the primary structure plays an important role in secondary and tertiary structures of Vpu TM in lipid bilayers and affects its ability to interact with channel blockers.
Figures
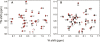



Similar articles
-
Drug sensitivity, drug-resistant mutations, and structures of three conductance domains of viral porins.Biochim Biophys Acta. 2011 Feb;1808(2):538-46. doi: 10.1016/j.bbamem.2010.07.015. Epub 2010 Jul 23. Biochim Biophys Acta. 2011. PMID: 20655872 Free PMC article. Review.
-
A single amino acid substitution within the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein renders simian-human immunodeficiency virus (SHIV(KU-1bMC33)) susceptible to rimantadine.Virology. 2006 May 10;348(2):449-61. doi: 10.1016/j.virol.2005.12.025. Epub 2006 Feb 3. Virology. 2006. PMID: 16458946
-
Three-dimensional structure of the transmembrane domain of Vpu from HIV-1 in aligned phospholipid bicelles.Biophys J. 2006 Oct 15;91(8):3032-42. doi: 10.1529/biophysj.106.087106. Epub 2006 Jul 21. Biophys J. 2006. PMID: 16861273 Free PMC article.
-
Substitution of the transmembrane domain of Vpu in simian-human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig-tailed macaques.Virology. 2006 Jan 20;344(2):541-59. doi: 10.1016/j.virol.2005.08.022. Epub 2005 Sep 30. Virology. 2006. PMID: 16199074
-
Structure-function correlates of Vpu, a membrane protein of HIV-1.FEBS Lett. 2003 Sep 18;552(1):47-53. doi: 10.1016/s0014-5793(03)00849-4. FEBS Lett. 2003. PMID: 12972151 Review.
Cited by
-
Comparative NMR studies demonstrate profound differences between two viroporins: p7 of HCV and Vpu of HIV-1.Biochim Biophys Acta. 2011 Feb;1808(2):554-60. doi: 10.1016/j.bbamem.2010.08.005. Epub 2010 Aug 18. Biochim Biophys Acta. 2011. PMID: 20727848 Free PMC article. Review.
-
Magic angle spinning NMR of viruses.Prog Nucl Magn Reson Spectrosc. 2015 Apr;86-87:21-40. doi: 10.1016/j.pnmrs.2015.02.003. Epub 2015 Feb 16. Prog Nucl Magn Reson Spectrosc. 2015. PMID: 25919197 Free PMC article. Review.
-
Advances in Viroporin Function and Structure: A Comparative Analysis of Alphavirus 6K with Well-Characterized Viroporins.Viruses. 2025 Jun 19;17(6):868. doi: 10.3390/v17060868. Viruses. 2025. PMID: 40573459 Free PMC article. Review.
-
Drug sensitivity, drug-resistant mutations, and structures of three conductance domains of viral porins.Biochim Biophys Acta. 2011 Feb;1808(2):538-46. doi: 10.1016/j.bbamem.2010.07.015. Epub 2010 Jul 23. Biochim Biophys Acta. 2011. PMID: 20655872 Free PMC article. Review.
-
The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives.Chem Rev. 2013 May 8;113(5):3516-604. doi: 10.1021/cr100264t. Epub 2013 Feb 25. Chem Rev. 2013. PMID: 23432396 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

