How similar are enzyme active site geometries derived from quantum mechanical theozymes to crystal structures of enzyme-inhibitor complexes? Implications for enzyme design
- PMID: 17766382
- PMCID: PMC2206971
- DOI: 10.1110/ps.072963707
How similar are enzyme active site geometries derived from quantum mechanical theozymes to crystal structures of enzyme-inhibitor complexes? Implications for enzyme design
Abstract
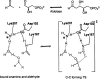
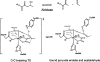
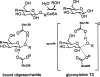
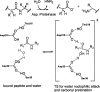
Quantum mechanical optimizations of theoretical enzymes (theozymes), which are predicted catalytic arrays of biological functionalities stabilizing a transition state, have been carried out for a set of nine diverse enzyme active sites. For each enzyme, the theozyme for the rate-determining transition state plus the catalytic groups modeled by side-chain mimics was optimized using B3LYP/6-31G(d) or, in one case, HF/3-21G(d) quantum mechanical calculations. To determine if the theozyme can reproduce the natural evolutionary catalytic geometry, the positions of optimized catalytic atoms, i.e., covalent, partial covalent, or stabilizing interactions with transition state atoms, are compared to the positions of the atoms in the X-ray crystal structure with a bound inhibitor. These structure comparisons are contrasted to computed substrate-active site structures surrounded by the same theozyme residues. The theozyme/transition structure is shown to predict geometries of active sites with an average RMSD of 0.64 A from the crystal structure, while the RMSD for the bound intermediate complexes are significantly higher at 1.42 A. The implications for computational enzyme design are discussed.
Figures
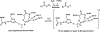
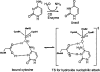
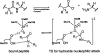
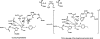

Similar articles
-
Quantum mechanical design of enzyme active sites.J Org Chem. 2008 Feb 1;73(3):889-99. doi: 10.1021/jo701974n. Epub 2008 Jan 8. J Org Chem. 2008. PMID: 18179229
-
Enzyme catalysis by hydrogen bonds: the balance between transition state binding and substrate binding in oxyanion holes.J Org Chem. 2010 Mar 19;75(6):1831-40. doi: 10.1021/jo901503d. J Org Chem. 2010. PMID: 20039621
-
The role of the putative catalytic base in the phosphoryl transfer reaction in a protein kinase: first-principles calculations.J Am Chem Soc. 2003 Aug 20;125(33):9926-7. doi: 10.1021/ja029618u. J Am Chem Soc. 2003. PMID: 12914447
-
Hybrid schemes based on quantum mechanics/molecular mechanics simulations goals to success, problems, and perspectives.Adv Protein Chem Struct Biol. 2011;85:81-142. doi: 10.1016/B978-0-12-386485-7.00003-X. Adv Protein Chem Struct Biol. 2011. PMID: 21920322 Review.
-
Theozymes and compuzymes: theoretical models for biological catalysis.Curr Opin Chem Biol. 1998 Dec;2(6):743-50. doi: 10.1016/s1367-5931(98)80112-9. Curr Opin Chem Biol. 1998. PMID: 9914196 Review.
Cited by
-
Acceleration of an aromatic Claisen rearrangement via a designed spiroligozyme catalyst that mimics the ketosteroid isomerase catalytic dyad.J Am Chem Soc. 2014 Mar 12;136(10):3817-27. doi: 10.1021/ja409214c. Epub 2014 Feb 27. J Am Chem Soc. 2014. PMID: 24456160 Free PMC article.
-
Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in epi-isozizaene synthase.Chem Sci. 2015 Apr 1;6(4):2347-2353. doi: 10.1039/c4sc03782k. Epub 2015 Feb 2. Chem Sci. 2015. PMID: 29308148 Free PMC article.
-
Structural reorganization and preorganization in enzyme active sites: comparisons of experimental and theoretically ideal active site geometries in the multistep serine esterase reaction cycle.J Am Chem Soc. 2008 Nov 19;130(46):15361-73. doi: 10.1021/ja803213p. Epub 2008 Oct 22. J Am Chem Soc. 2008. PMID: 18939839 Free PMC article.
-
SABER: a computational method for identifying active sites for new reactions.Protein Sci. 2012 May;21(5):697-706. doi: 10.1002/pro.2055. Protein Sci. 2012. PMID: 22492397 Free PMC article.
-
Investigations on recyclisation and hydrolysis in avibactam mediated serine β-lactamase inhibition.Org Biomol Chem. 2016 Apr 26;14(17):4116-28. doi: 10.1039/c6ob00353b. Org Biomol Chem. 2016. PMID: 27072755 Free PMC article.
References
-
- Arnó M. and Domingo, L.R. 2001. Using theozymes for designing transition-state analogs for the intramolecular aldol reaction of δ-diketones. Int. J. Quantum Chem. 83 338–347.
-
- Arnó M. and Domingo, L.R. 2003. Theozyme for antibody aldolases. Characterization of the transition-state analogue. Org. Biomol. Chem. 1 637–643. - PubMed
-
- Bartlett G.J., Porter, G.T., Borkakoti, N., and Thornton, J.M. 2002. Analysis of catalytic residues in enzyme active sites. J. Mol. Biol. 324 105–121. - PubMed
-
- Beveridge A.J. 1998. A theoretical study of the initial stages of catalysis in the aspartic proteinases. J. Mol. Struct. 453 275–291.
-
- Blanchard J.E. and Withers, S.G. 2001. Rapid screening of the aglycone specificity of glycosidases: Applications to enzymatic synthesis of oligosaccaharides. Chem. Biol. 8 627–633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

