New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots
- PMID: 17766446
- PMCID: PMC2074954
- DOI: 10.1128/AEM.00405-07
New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots
Abstract
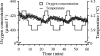
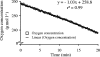
A new method of respiration rate measurement based on oxygen luminescence quenching in sensor spots was evaluated for the first time for aquatic bacterial communities. The commonly used Winkler and Clark electrode methods to quantify oxygen concentration both require long incubation times, and the latter additionally causes signal drift due to oxygen consumption at the cathode. The sensor spots proved to be advantageous over those methods in terms of precise and quick oxygen measurements in natural bacterial communities, guaranteeing a respiration rate estimate during a time interval short enough to neglect variations in organism composition, abundance, and activity. Furthermore, no signal drift occurs during measurements, and respiration rate measurements are reliable even at low temperatures and low oxygen consumption rates. Both a natural bacterioplankton sample and a bacterial isolate from a eutrophic river were evaluated in order to optimize the new method for aquatic microorganisms. A minimum abundance of 2.2 x 10(6) respiring cells ml(-1) of a bacterial isolate was sufficient to obtain a distinct oxygen depletion signal within 20 min at 20 degrees C with the new oxygen sensor spot method. Thus, a culture of a bacterial isolate from a eutrophic river (OW 144; 20 x 10(6) respiring bacteria ml(-1)) decreased the oxygen saturation about 8% within 20 min. The natural bacterioplankton sample respired 2.8% from initially 94% oxygen-saturated water in 30 min. During the growth season in 2005, the planktonic community of a eutrophic river consumed between 0.7 and 15.6 micromol O(2) liter(-1) h(-1). The contribution of bacterial respiration to the total plankton community oxygen consumption varied seasonally between 11 and 100%.
Figures






Similar articles
-
Dynamics and estimates of growth and loss rates of bacterioplankton in a temperate freshwater system.FEMS Microbiol Ecol. 2006 Oct;58(1):23-32. doi: 10.1111/j.1574-6941.2006.00145.x. FEMS Microbiol Ecol. 2006. PMID: 16958905
-
[The number and respiratory intensity of bacterioplankton in the South Ural lakes].Mikrobiologiia. 1976 Mar-Apr;45(2):358-64. Mikrobiologiia. 1976. PMID: 933887 Russian.
-
INT (2-(4-Iodophenyl)-3-(4-Nitrophenyl)-5-(Phenyl) Tetrazolium Chloride) Is Toxic to Prokaryote Cells Precluding Its Use with Whole Cells as a Proxy for In Vivo Respiration.Microb Ecol. 2015 Nov;70(4):1004-11. doi: 10.1007/s00248-015-0626-3. Epub 2015 May 20. Microb Ecol. 2015. PMID: 25991603
-
Aquatic bacterial diversity: Magnitude, dynamics, and controlling factors.Microb Pathog. 2017 Mar;104:39-47. doi: 10.1016/j.micpath.2017.01.016. Epub 2017 Jan 5. Microb Pathog. 2017. PMID: 28065819 Review.
-
Regulation of bacterial assemblages in oligotrophic plankton systems: results from experimental and empirical approaches.Antonie Van Leeuwenhoek. 2002 Aug;81(1-4):435-52. doi: 10.1023/a:1020578418898. Antonie Van Leeuwenhoek. 2002. PMID: 12448741 Review.
Cited by
-
Bacterial activity and bacterioplankton diversity in the eutrophic River Warnow--direct measurement of bacterial growth efficiency and its effect on carbon utilization.Microb Ecol. 2011 Jan;61(1):190-200. doi: 10.1007/s00248-010-9729-z. Epub 2010 Jul 31. Microb Ecol. 2011. PMID: 20676625
-
Respiration, Production, and Growth Efficiency of Marine Pelagic Fungal Isolates.J Fungi (Basel). 2023 Mar 28;9(4):417. doi: 10.3390/jof9040417. J Fungi (Basel). 2023. PMID: 37108872 Free PMC article.
-
Contrasting effects of ultraviolet radiation on the growth efficiency of freshwater bacteria.Aquat Ecol. 2011 Mar;45(1):125-136. doi: 10.1007/s10452-010-9341-9. Aquat Ecol. 2011. PMID: 21516253 Free PMC article.
-
UV-induced effects on growth, photosynthetic performance and sunscreen contents in different populations of the green alga Klebsormidium fluitans (Streptophyta) from alpine soil crusts.Microb Ecol. 2014 Feb;67(2):327-40. doi: 10.1007/s00248-013-0317-x. Microb Ecol. 2014. PMID: 24233286
-
Pseudomonas aeruginosa Aggregate Formation in an Alginate Bead Model System Exhibits In Vivo-Like Characteristics.Appl Environ Microbiol. 2017 Apr 17;83(9):e00113-17. doi: 10.1128/AEM.00113-17. Print 2017 May 1. Appl Environ Microbiol. 2017. PMID: 28258141 Free PMC article.
References
-
- Arain, S., G. T. John, C. Krause, J. Gerlach, O. S. Wolfbeis, and I. Klimant. 2006. Characterization of microtiter plates with integrated optical sensors for oxygen and pH, and their applications to enzyme activity screening, respirometry, and toxicological assays. Sens. Actuators B 113:639-648.
-
- Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer Reil, and F. Thingstad. 1983. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10:257-263.
-
- Bernardi, R., G. Giussani, M. Manca, and D. Ruggiu. 1990. Trophic status and the pelagic system in Lago Maggiore. Hydrobiologia 191:1-8.
-
- Boyd, T. J., and A. F. Carlucci. 1994. Use of a H-3 labeled substrate to measure microbial biodegradation in marine waters. J. Microbiol. Methods 20:11-22.
-
- Carignan, R., A. M. Blais, and C. Vis. 1998. Measurement of primary production and community respiration in oligotrophic lakes using the Winkler method. Can. J. Fish. Aquat. Sci. 55:1078-1084.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

