Tag team action at the synapse
- PMID: 17767192
- PMCID: PMC1973957
- DOI: 10.1038/sj.embor.7401051
Tag team action at the synapse
Abstract
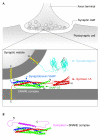
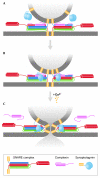
Communication between neurons relies on chemical synapses and the release of neurotransmitters into the synaptic cleft. Neurotransmitter release is an exquisitely regulated membrane fusion event that requires the linking of an electrical nerve stimulus to Ca(2+) influx, which leads to the fusion of neurotransmitter-filled vesicles with the cell membrane. The timing of neurotransmitter release is controlled through the regulation of the soluble N-ethylmaleimide sensitive factor attachment receptor (SNARE) proteins-the core of the membrane fusion machinery. Assembly of the fusion-competent SNARE complex is regulated by several neuronal proteins, including complexin and the Ca(2+)-sensor synaptotagmin. Both complexin and synaptotagmin bind directly to SNAREs, but their mechanism of action has so far remained unclear. Recent studies revealed that synaptotagmin-Ca(2+) and complexin collaborate to regulate membrane fusion. These compelling new results provide a molecular mechanistic insight into the functions of both proteins: complexin 'clamps' the SNARE complex in a pre-fusion intermediate, which is then released by the action of Ca(2+)-bound synaptotagmin to trigger rapid fusion.
Figures


Similar articles
-
A clamping mechanism involved in SNARE-dependent exocytosis.Science. 2006 Aug 4;313(5787):676-80. doi: 10.1126/science.1129450. Epub 2006 Jun 22. Science. 2006. PMID: 16794037
-
Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes.Biochemistry. 2009 Feb 3;48(4):657-9. doi: 10.1021/bi801962d. Biochemistry. 2009. PMID: 19128031 Free PMC article.
-
The Core Complex of the Ca2+-Triggered Presynaptic Fusion Machinery.J Mol Biol. 2023 Jan 15;435(1):167853. doi: 10.1016/j.jmb.2022.167853. Epub 2022 Oct 13. J Mol Biol. 2023. PMID: 36243149 Free PMC article. Review.
-
Ca2+-dependent release of synaptotagmin-1 from the SNARE complex on phosphatidylinositol 4,5-bisphosphate-containing membranes.Elife. 2020 Aug 18;9:e57154. doi: 10.7554/eLife.57154. Elife. 2020. PMID: 32808925 Free PMC article.
-
Molecular Mechanisms Underlying Neurotransmitter Release.Annu Rev Biophys. 2022 May 9;51:377-408. doi: 10.1146/annurev-biophys-111821-104732. Epub 2022 Feb 15. Annu Rev Biophys. 2022. PMID: 35167762 Free PMC article. Review.
Cited by
-
Expanding the substantial interactome of NEMO using protein microarrays.PLoS One. 2010 Jan 20;5(1):e8799. doi: 10.1371/journal.pone.0008799. PLoS One. 2010. PMID: 20098747 Free PMC article.
-
Airway mucus: the good, the bad, the sticky.Pharmacol Ther. 2009 Mar;121(3):332-48. doi: 10.1016/j.pharmthera.2008.11.001. Epub 2008 Nov 18. Pharmacol Ther. 2009. PMID: 19059283 Free PMC article. Review.
-
Histidine-tag-directed chromophores for tracer analyses in the analytical ultracentrifuge.Methods. 2011 May;54(1):31-8. doi: 10.1016/j.ymeth.2010.12.033. Epub 2010 Dec 25. Methods. 2011. PMID: 21187151 Free PMC article. Review.
-
Pulling force generated by interacting SNAREs facilitates membrane hemifusion.Integr Biol (Camb). 2009 Apr;1(4):301-10. doi: 10.1039/b900685k. Epub 2009 Feb 26. Integr Biol (Camb). 2009. PMID: 20023730 Free PMC article.
-
Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells.J Biol Chem. 2009 Jul 17;284(29):19445-51. doi: 10.1074/jbc.M109.002550. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473977 Free PMC article.
References
-
- Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J (2006) Close membrane–membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol 13: 209–217 - PubMed
-
- Bai J, Chapman ER (2004) The C2 domains of synaptotagmin—partners in exocytosis. Trends Biochem Sci 29: 143–151 - PubMed
-
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116: 153–166 - PubMed
-
- Bracher A, Kadlec J, Betz H, Weissenhorn W (2002) X-ray structure of a neuronal complexin–SNARE complex from squid. J Biol Chem 277: 26517–26523 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

