Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus
- PMID: 17767488
- PMCID: PMC2295210
- DOI: 10.1111/j.1460-9568.2007.05751.x
Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus
Abstract
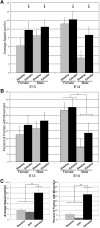
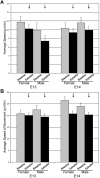
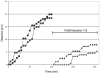
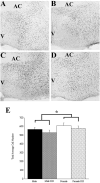
The preoptic area-anterior hypothalamus (POA-AH) is characterized by sexually dimorphic features in a number of vertebrates and is a key region of the forebrain for regulating physiological responses and sexual behaviours. Using live-cell fluorescence video microscopy with organotypic brain slices, the current study examined sex differences in the movement characteristics of neurons expressing yellow fluorescent protein (YFP) driven by the Thy-1 promoter. Cells in slices from embryonic day 14 (E14), but not E13, mice displayed significant sex differences in their basal neuronal movement characteristics. Exposure to 10 nm estradiol-17beta (E2), but not 100 nm dihydrotestosterone, significantly altered cell movement characteristics within minutes of exposure, in a location-specific manner. E2 treatment decreased the rate of motion of cells located in the dorsal POA-AH but increased the frequency of movement in cells located more ventrally. These effects were consistent across age and sex. To further determine whether early-developing sex differences in the POA-AH depend upon gonadal steroids, we examined cell positions in mice with a disruption of the steroidogenic factor-1 gene, in which gonads do not form. An early-born cohort of cells were labelled with the mitotic indicator bromodeoxyuridine (BrdU) on E11. More cells were found in the POA-AH of females than males on the day of birth (P0) regardless of gonadal status. These results support the hypothesis that estrogen partially contributes to brain sexual dimorphism through its influence on cell movements during development. Estrogen's influence may be superimposed upon a pre-existing genetic bias.
Figures


Similar articles
-
Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret.J Neurobiol. 1996 Jul;30(3):315-28. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. J Neurobiol. 1996. PMID: 8807525
-
Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice.J Neurobiol. 1999 Nov 5;41(2):252-66. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. J Neurobiol. 1999. PMID: 10512982
-
Large somal size is associated with the expression of galanin but not with neuronal birthdate in the sexually dimorphic male nucleus of ferret's preoptic area/anterior hypothalamus.Neuroendocrinology. 1998 Oct;68(4):235-43. doi: 10.1159/000054371. Neuroendocrinology. 1998. PMID: 9772338
-
Estrogenic control of preoptic area development in a carnivore, the ferret.Cell Mol Neurobiol. 1996 Apr;16(2):117-28. doi: 10.1007/BF02088171. Cell Mol Neurobiol. 1996. PMID: 8743964 Free PMC article. Review.
-
Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats.J Neuroendocrinol. 2009 Mar;21(4):370-6. doi: 10.1111/j.1365-2826.2009.01855.x. J Neuroendocrinol. 2009. PMID: 19226350 Review.
Cited by
-
Developmental profile and sexually dimorphic expression of kiss1 and kiss1r in the fetal mouse brain.Front Endocrinol (Lausanne). 2013 Oct 11;4:140. doi: 10.3389/fendo.2013.00140. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 24130552 Free PMC article.
-
Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system.Front Neuroendocrinol. 2016 Jan;40:67-86. doi: 10.1016/j.yfrne.2016.01.001. Epub 2016 Jan 11. Front Neuroendocrinol. 2016. PMID: 26790970 Free PMC article. Review.
-
Brain sex differences and hormone influences: a moving experience?J Neuroendocrinol. 2009 Mar;21(4):387-92. doi: 10.1111/j.1365-2826.2009.01834.x. J Neuroendocrinol. 2009. PMID: 19207813 Free PMC article. Review.
-
Sexual differentiation of the rodent brain: dogma and beyond.Front Neurosci. 2012 Feb 21;6:26. doi: 10.3389/fnins.2012.00026. eCollection 2012. Front Neurosci. 2012. PMID: 22363256 Free PMC article.
-
Research Resource: The Dexamethasone Transcriptome in Hypothalamic Embryonic Neural Stem Cells.Mol Endocrinol. 2016 Jan;30(1):144-54. doi: 10.1210/me.2015-1258. Epub 2015 Nov 25. Mol Endocrinol. 2016. PMID: 26606517 Free PMC article.
References
-
- Acconcia F, Barnes CJ, Kumar R. Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology. 2006;147:1203–1212. - PubMed
-
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. - PubMed
-
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. - PubMed
-
- Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

