Fluorescence staining of live cyanobacterial cells suggest non-stringent chromosome segregation and absence of a connection between cytoplasmic and thylakoid membranes
- PMID: 17767716
- PMCID: PMC2040150
- DOI: 10.1186/1471-2121-8-39
Fluorescence staining of live cyanobacterial cells suggest non-stringent chromosome segregation and absence of a connection between cytoplasmic and thylakoid membranes
Abstract
Background: In spite of their abundance and importance, little is known about cyanobacterial cell biology and their cell cycle. During each cell cycle, chromosomes must be separated into future daughter cells, i.e. into both cell halves, which in many bacteria is achieved by an active machinery that operates during DNA replication. Many cyanobacteria contain multiple identical copies of the chromosome, but it is unknown how chromosomes are segregated into future daughter cells, and if an active or passive mechanism is operative. In addition to an outer and an inner cell membrane, cyanobacteria contain internal thylakoid membranes that carry the active photosynthetic machinery. It is unclear whether thylakoid membranes are invaginations of the inner cell membrane, or an independent membrane system.
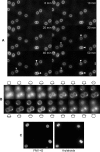
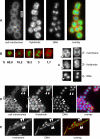
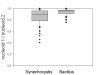
Results: We have used different fluorescent dyes to study the organization of chromosomes and of cell and thylakoid membranes in live cyanobacterial cells. FM1-43 stained the outer and inner cytoplasmic membranes but did not enter the interior of the cell. In contrast, thylakoid membranes in unicellular Synechocystis cells became visible through a membrane-permeable stain only. Furthermore, continuous supply of the fluorescent dye FM1-43 resulted in the formation of one to four intracellular fluorescent structures in Synechocystis cells, within occurred within 30 to 60 minutes, and may represent membrane vesicles. Using fluorescent DNA stains, we found that Synechocystis genomic DNA is compacted in the cell centre that is devoid of thylakoid membranes. Nucleoids segregated very late in the cell cycle, just before complete closing of the division septum. In striking contrast to Bacillus subtilis, which possesses an active chromosome segregation machinery, fluorescence intensity of stained nucleoids differed considerably between the two Synechocystis daughter cells soon after cell division.
Conclusion: Our experiments strongly support the idea that the cytoplasmic and thylakoid membranes are not directly connected, but separate entities, in unicellular cyanobacteria. Our findings suggest that a transport system may exist between the cytoplasmic membrane and thylakoids, which could mediate the extension of thylakoid membranes and possibly also protein transport from the cytoplasmic membrane to thylakoid membranes. The cell cycle studies in Synechocystis sp. PCC 6803 show that the multiple chromosome copies per cell segregate very late in the cell cycle and in a much less stringent manner than in B. subtilis cells, indicating that chromosomes may become segregated randomly and in a passive fashion, possibly through constriction of the division septum.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

