Inhibition of striatal dopamine release by CB1 receptor activation requires nonsynaptic communication involving GABA, H2O2, and KATP channels
- PMID: 17767979
- PMCID: PMC2904528
- DOI: 10.1016/j.neuint.2007.07.014
Inhibition of striatal dopamine release by CB1 receptor activation requires nonsynaptic communication involving GABA, H2O2, and KATP channels
Abstract
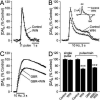
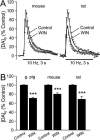
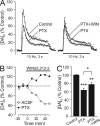
The main psychoactive component of marijuana, Delta9-tetrahydrocannabinol (THC), acts in the CNS via type 1 cannabinoid receptors (CB1Rs). The behavioral consequences of THC or synthetic CB1R agonists include suppression of motor activity. One explanation for movement suppression might be inhibition of striatal dopamine (DA) release by CB1Rs, which are densely localized in motor striatum; however, data from previous studies are inconclusive. Here we examined the effect of CB1R activation on locally evoked DA release monitored with carbon-fiber microelectrodes and fast-scan cyclic voltammetry in striatal slices. Consistent with previous reports, DA release evoked by a single stimulus pulse was unaffected by WIN55,212-2, a cannabinoid receptor agonist. However, when DA release was evoked by a train of stimuli, WIN55,212-2 caused a significant decrease in evoked extracellular DA concentration ([DA]o), implicating the involvement of local striatal circuitry, with similar suppression seen in guinea pig, rat, and mouse striatum. Pulse-train evoked [DA]o was not altered by either AM251, an inverse CB1R agonist, or VCHSR1, a neutral antagonist, indicating the absence of DA release regulation by endogenous cannabinoids with the stimulation protocol used. However, both CB1R antagonists prevented and reversed suppression of evoked [DA]o by WIN55,212-2. The effect of WIN55,212-2 was also prevented by picrotoxin, a GABAA receptor antagonist, and by catalase, a metabolizing enzyme for hydrogen peroxide (H2O2). Furthermore, blockade of ATP-sensitive K+ (KATP) channels by tolbutamide or glybenclamide prevented the effect of WIN55,212-2 on DA release. Together, these data indicate that suppression of DA release by CB1R activation within striatum occurs via a novel nonsynaptic mechanism that involves GABA release inhibition, increased generation of the diffusible messenger H2O2, and activation of KATP channels to inhibit DA release. In addition, the findings suggest a possible physiological substrate for the motor effects of cannabinoid agonist administration.
Figures
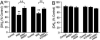
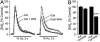
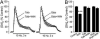
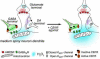
Similar articles
-
Axonal Modulation of Striatal Dopamine Release by Local γ-Aminobutyric Acid (GABA) Signalling.Cells. 2021 Mar 23;10(3):709. doi: 10.3390/cells10030709. Cells. 2021. PMID: 33806845 Free PMC article. Review.
-
Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release.Proc Natl Acad Sci U S A. 2003 Sep 30;100(20):11729-34. doi: 10.1073/pnas.1834314100. Epub 2003 Sep 17. Proc Natl Acad Sci U S A. 2003. PMID: 13679582 Free PMC article.
-
Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion.J Neurosci. 2005 Oct 26;25(43):10029-40. doi: 10.1523/JNEUROSCI.2652-05.2005. J Neurosci. 2005. PMID: 16251452 Free PMC article.
-
Comparing adenosine A2A receptor modulation of cannabinoid CB1 receptor-mediated inhibition of GABA and glutamate release in rodent striatal nerve terminals.Eur J Neurosci. 2025 Jan;61(1):e16642. doi: 10.1111/ejn.16642. Eur J Neurosci. 2025. PMID: 39777752
-
Classification of H₂O₂as a neuromodulator that regulates striatal dopamine release on a subsecond time scale.ACS Chem Neurosci. 2012 Dec 19;3(12):991-1001. doi: 10.1021/cn300130b. Epub 2012 Nov 8. ACS Chem Neurosci. 2012. PMID: 23259034 Free PMC article. Review.
Cited by
-
Endocannabinoid Modulation of Nucleus Accumbens Microcircuitry and Terminal Dopamine Release.Front Synaptic Neurosci. 2021 Aug 23;13:734975. doi: 10.3389/fnsyn.2021.734975. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34497503 Free PMC article. Review.
-
The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor-independent mechanism.J Neurochem. 2010 Mar;112(6):1454-64. doi: 10.1111/j.1471-4159.2009.06557.x. Epub 2009 Dec 24. J Neurochem. 2010. PMID: 20050977 Free PMC article.
-
Effects of Cannabinoid Exposure during Neurodevelopment on Future Effects of Drugs of Abuse: A Preclinical Perspective.Int J Mol Sci. 2021 Sep 15;22(18):9989. doi: 10.3390/ijms22189989. Int J Mol Sci. 2021. PMID: 34576153 Free PMC article. Review.
-
Axonal Modulation of Striatal Dopamine Release by Local γ-Aminobutyric Acid (GABA) Signalling.Cells. 2021 Mar 23;10(3):709. doi: 10.3390/cells10030709. Cells. 2021. PMID: 33806845 Free PMC article. Review.
-
Cannabinoid CB1 Receptors Are Expressed in a Subset of Dopamine Neurons and Underlie Cannabinoid-Induced Aversion, Hypoactivity, and Anxiolytic Effects in Mice.J Neurosci. 2023 Jan 18;43(3):373-385. doi: 10.1523/JNEUROSCI.1493-22.2022. Epub 2022 Dec 14. J Neurosci. 2023. PMID: 36517243 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. - PubMed
-
- Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxidants and Redox Signaling. 2007;9:219–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

