Crystallization and X-ray structure of full-length recombinant human butyrylcholinesterase
- PMID: 17768338
- PMCID: PMC2376307
- DOI: 10.1107/S1744309107037335
Crystallization and X-ray structure of full-length recombinant human butyrylcholinesterase
Abstract
Human butyrylcholinesterase (BChE) has been shown to function as an endogenous scavenger of diverse poisons. BChE is a 340 kDa tetrameric glycoprotein that is present in human serum at a concentration of 5 mg l(-1). The well documented therapeutic effects of BChE on cocaine toxicity and organophosphorus agent poisoning has increased the need for effective methods of producing recombinant therapeutic BChE. In order to be therapeutically useful, BChE must have a long circulatory residence time or associate as tetramers. Full-length recombinant BChE produced in Chinese hamster ovary (CHO) cells or human embryonic kidney cells has been shown to associate as monomers, with a shorter circulatory residence time than the naturally occurring tetrameric serum protein. Based on the preceding observation as well as the need to develop novel methodologies to facilitate the mass production of therapeutic recombinant BChE, studies have been initiated to determine the structural basis of tetramer formation. Towards these ends, full-length monomeric recombinant BChE has been crystallized for the first time. A 2.8 A X-ray structure was solved in space group P42(1)2, with unit-cell parameters a = b = 156, c = 146 A.
Figures

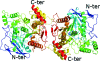

Similar articles
-
Cocrystallization studies of full-length recombinant butyrylcholinesterase (BChE) with cocaine.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Apr 1;67(Pt 4):434-7. doi: 10.1107/S1744309111004805. Epub 2011 Mar 24. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21505234 Free PMC article.
-
Effect of polyethylene glycol modification on the circulatory stability and immunogenicity of recombinant human butyrylcholinesterase.Chem Biol Interact. 2008 Sep 25;175(1-3):255-60. doi: 10.1016/j.cbi.2008.05.020. Epub 2008 May 21. Chem Biol Interact. 2008. PMID: 18603232
-
Human butyrylcholinesterase produced in insect cells: huprine-based affinity purification and crystal structure.FEBS J. 2012 Aug;279(16):2905-16. doi: 10.1111/j.1742-4658.2012.08672.x. Epub 2012 Jul 12. FEBS J. 2012. PMID: 22726956
-
Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses.Pharmacol Ther. 2015 Apr;148:34-46. doi: 10.1016/j.pharmthera.2014.11.011. Epub 2014 Nov 20. Pharmacol Ther. 2015. PMID: 25448037 Review.
-
Butyrylcholinesterase deficiency.Ann Biol Clin (Paris). 2016 Jun 1;74(3):279-85. doi: 10.1684/abc.2016.1141. Ann Biol Clin (Paris). 2016. PMID: 27237801 Review. English.
Cited by
-
Evaluation of Anticholinesterase Activity of the Fungicides Mefentrifluconazole and Pyraclostrobin.Int J Mol Sci. 2024 Jun 7;25(12):6310. doi: 10.3390/ijms25126310. Int J Mol Sci. 2024. PMID: 38928014 Free PMC article.
-
Hot Spots for Protein Partnerships at the Surface of Cholinesterases and Related α/β Hydrolase Fold Proteins or Domains-A Structural Perspective.Molecules. 2017 Dec 23;23(1):35. doi: 10.3390/molecules23010035. Molecules. 2017. PMID: 29295471 Free PMC article. Review.
-
Cholesterol Oxime Olesoxime Assessed as a Potential Ligand of Human Cholinesterases.Biomolecules. 2024 May 15;14(5):588. doi: 10.3390/biom14050588. Biomolecules. 2024. PMID: 38785995 Free PMC article.
-
Vitamin B3-Based Biologically Active Compounds as Inhibitors of Human Cholinesterases.Int J Mol Sci. 2020 Oct 29;21(21):8088. doi: 10.3390/ijms21218088. Int J Mol Sci. 2020. PMID: 33138280 Free PMC article.
-
Design and evaluation of selective butyrylcholinesterase inhibitors based on Cinchona alkaloid scaffold.PLoS One. 2018 Oct 5;13(10):e0205193. doi: 10.1371/journal.pone.0205193. eCollection 2018. PLoS One. 2018. PMID: 30289893 Free PMC article.
References
-
- Altamirano, C. V. & Lockridge, O. (1999a). Biochemistry, 38, 13414–13422. - PubMed
-
- Altamirano, C. V. & Lockridge, O. (1999b). Chem. Biol. Interact.119–120, 53–60. - PubMed
-
- Bon, S., Coussen, F. & Massoulie, J. (1997). J. Biol. Chem.272, 3016–3021. - PubMed
-
- Bourne, Y., Grassi, J., Bougis, P. E. & Marchot, P. (1999). J. Biol. Chem.274, 30370–30376. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

