Introduction of functional chimeric E/L-selectin by RNA electroporation to target dendritic cells from blood to lymph nodes
- PMID: 17768622
- PMCID: PMC11041385
- DOI: 10.1007/s00262-007-0385-1
Introduction of functional chimeric E/L-selectin by RNA electroporation to target dendritic cells from blood to lymph nodes
Abstract
Background: Inefficient migration of dendritic cells (DC) to regional lymph nodes (LN) upon intracutaneous injection is a major obstacle for effective DC vaccination. Intravenous vaccination is unfavorable, because DC cannot migrate directly from the blood into LN.
Methods: To enable human monocyte-derived (mo)DC to enter LN directly from the blood, we manipulated them by RNA electroporation to express a human chimeric E/L-selectin (CD62E/CD62L) protein, which binds to peripheral node addressin expressed on high endothelial venules.
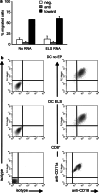
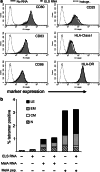
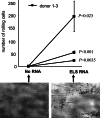
Results: Transfection efficiency exceeded 95%, and high E/L-selectin surface expression was detected for >48 h. E/L-selectin RNA-transfected DC displayed an identical mature DC phenotype as mock-transfected DC. Furthermore, E/L-selectin-transfected DC maintained their normal CCR7-mediated migration capacity, and their ability to prime and expand functional cytotoxic T cells recognizing MelanA. Most importantly, E/L-selectin-RNA-transfected DC gained the capability to attach to and roll on sialyl-Lewis(X) in vitro.
Outlook: The presented strategy can be readily translated into the clinic, as it involves no stable genetic manipulation or viral transformation, and allows targeting of a large number of LN.
Figures

Similar articles
-
Gene therapy to target dendritic cells from blood to lymph nodes.Gene Ther. 2003 Aug;10(17):1479-86. doi: 10.1038/sj.gt.3302008. Gene Ther. 2003. PMID: 12900763
-
P- and E-selectins recognize sialyl 6-sulfo Lewis X, the recently identified L-selectin ligand.Biochem Biophys Res Commun. 2000 Nov 11;278(1):90-6. doi: 10.1006/bbrc.2000.3768. Biochem Biophys Res Commun. 2000. PMID: 11071860
-
Effective clinical-scale production of dendritic cell vaccines by monocyte elutriation directly in medium, subsequent culture in bags and final antigen loading using peptides or RNA transfection.J Immunother. 2007 Sep;30(6):663-74. doi: 10.1097/CJI.0b013e3180ca7cd6. J Immunother. 2007. PMID: 17667530
-
Sulfated L-selectin ligands as a therapeutic target in chronic inflammation.Trends Immunol. 2006 Dec;27(12):559-65. doi: 10.1016/j.it.2006.10.007. Epub 2006 Oct 17. Trends Immunol. 2006. PMID: 17049924 Review.
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
Cited by
-
CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential.Clin Exp Immunol. 2009 Feb;155(2):249-56. doi: 10.1111/j.1365-2249.2008.03820.x. Epub 2008 Nov 24. Clin Exp Immunol. 2009. PMID: 19040609 Free PMC article. Review.
-
Induction of CD4(+) Regulatory and Polarized Effector/helper T Cells by Dendritic Cells.Immune Netw. 2016 Feb;16(1):13-25. doi: 10.4110/in.2016.16.1.13. Epub 2016 Feb 25. Immune Netw. 2016. PMID: 26937228 Free PMC article. Review.
-
Delivery strategies of cancer immunotherapy: recent advances and future perspectives.J Hematol Oncol. 2019 Nov 28;12(1):126. doi: 10.1186/s13045-019-0817-3. J Hematol Oncol. 2019. PMID: 31779642 Free PMC article. Review.
-
Ribonucleic Acid Engineering of Dendritic Cells for Therapeutic Vaccination: Ready 'N Able to Improve Clinical Outcome?Cancers (Basel). 2020 Jan 27;12(2):299. doi: 10.3390/cancers12020299. Cancers (Basel). 2020. PMID: 32012714 Free PMC article. Review.
-
A One-Armed Phase I Dose Escalation Trial Design: Personalized Vaccination with IKKβ-Matured, RNA-Loaded Dendritic Cells for Metastatic Uveal Melanoma.Front Immunol. 2022 Feb 4;13:785231. doi: 10.3389/fimmu.2022.785231. eCollection 2022. Front Immunol. 2022. PMID: 35185883 Free PMC article.
References
-
- Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

