Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia
- PMID: 17784877
- PMCID: PMC8095593
- DOI: 10.1111/j.1750-3639.2007.00088.x
Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia
Abstract
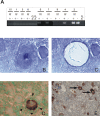
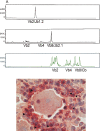
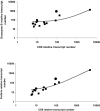
The latent persistence of herpes simplex virus type 1 (HSV-1) in human trigeminal ganglia (TG) is accompanied by a chronic CD8 T-cell infiltrate. The focus of the current work was to look for HSV-1 transcription activity as a potential trigger of the immune response and to characterize the immune cell infiltrates by this feature. We combined in situ hybridization, laser cutting microscopy, and single cell RT-PCR to demonstrate the expression of the HSV-1 immediate early (IE) genes ICP0 and ICP4 in human trigeminal neurons. Using CDR3 spectratyping, we showed that the infiltrating T-cells are clonally expanded, indicating an antigen-driven immune response. Moreover, the persisting CD8+ T-cells had features of the memory effector phenotype. The voltage-gated potassium channel Kv1.3, a marker of chronic activated memory effector cells, and the chemokines CCL5 and CXCL10 were expressed by a subpopulation of infiltrating cells. The corresponding chemokine receptors CCR5 and CXCR3 were co-expressed on virtually all CD8 T-cells. In addition, T-cells expressed granzymes and perforin. In contrast to animal models of HSV-1 latency, hardly any FoxP3-positive regulatory T-cells were detected in human TG. Thus, HSV-1 IE genes are expressed in human TG and the infiltrating T-cells bear several characteristics that suggest viral antigenic stimulation.
Figures


References
-
- Chen SH, Garber DA, Schaffer PA, Knipe DM, Coen DM (2000) Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology 278:207–216. - PubMed
-
- Cohrs RJ, Laguardia JJ, Gilden D (2005) Distribution of latent herpes simplex virus type‐1 and varicella zoster virus DNA in human trigeminal Ganglia. Virus Genes 31:223–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

