Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1
- PMID: 17785433
- PMCID: PMC2169056
- DOI: 10.1128/MCB.00440-07
Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1
Abstract
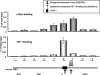
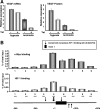
Hypoxia is a pervasive microenvironmental factor that affects normal development as well as tumor progression. In most normal cells, hypoxia stabilizes hypoxia-inducible transcription factors (HIFs), particularly HIF-1, which activates genes involved in anaerobic metabolism and angiogenesis. As hypoxia signals a cellular deprivation state, HIF-1 has also been reported to counter the activity of MYC, which encodes a transcription factor that drives cell growth and proliferation. Since many human cancers express dysregulated MYC, we sought to determine whether HIF-1 would in fact collaborate with dysregulated MYC rather countering its function. Here, using the P493-6 Burkitt's lymphoma model with an inducible MYC, we demonstrate that HIF-1 cooperates with dysregulated c-Myc to promote glycolysis by induction of hexokinase 2, which catalyzes the first step of glycolysis, and pyruvate dehydrogenase kinase 1, which inactivates pyruvate dehydrogenase and diminishes mitochondrial respiration. We also found the collaborative induction of vascular endothelial growth factor (VEGF) by HIF-1 and dysregulated c-Myc. This study reports the previously unsuspected collaboration between HIF-1 and dysregulated MYC and thereby provides additional insights into the regulation of VEGF and the Warburg effect, which describes the propensity for cancer cells to convert glucose to lactate.
Figures



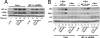
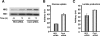


Similar articles
-
The interplay between MYC and HIF in the Warburg effect.Ernst Schering Found Symp Proc. 2007;(4):35-53. doi: 10.1007/2789_2008_088. Ernst Schering Found Symp Proc. 2007. PMID: 18811052 Review.
-
A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways.Cell Cycle. 2010 May;9(9):1722-8. doi: 10.4161/cc.9.9.11358. Epub 2010 May 1. Cell Cycle. 2010. PMID: 20404562 Free PMC article. Review.
-
Hypoxic regulation of vascular endothelial growth factor through the induction of phosphatidylinositol 3-kinase/Rho/ROCK and c-Myc.J Biol Chem. 2006 May 19;281(20):13957-63. doi: 10.1074/jbc.M511763200. Epub 2006 Mar 16. J Biol Chem. 2006. PMID: 16543245
-
TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells.Mol Cancer. 2013 Jul 17;12:78. doi: 10.1186/1476-4598-12-78. Mol Cancer. 2013. PMID: 23866118 Free PMC article.
-
Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance.J Biol Chem. 2008 Oct 17;283(42):28106-14. doi: 10.1074/jbc.M803508200. Epub 2008 Aug 21. J Biol Chem. 2008. PMID: 18718909 Free PMC article.
Cited by
-
Metabolic flux rewiring in mammalian cell cultures.Curr Opin Biotechnol. 2013 Dec;24(6):1108-15. doi: 10.1016/j.copbio.2013.04.016. Epub 2013 May 28. Curr Opin Biotechnol. 2013. PMID: 23726154 Free PMC article. Review.
-
CDK7 inhibitor THZ1 enhances antiPD-1 therapy efficacy via the p38α/MYC/PD-L1 signaling in non-small cell lung cancer.J Hematol Oncol. 2020 Jul 20;13(1):99. doi: 10.1186/s13045-020-00926-x. J Hematol Oncol. 2020. PMID: 32690037 Free PMC article.
-
Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis.Nat Metab. 2020 Mar;2(3):256-269. doi: 10.1038/s42255-020-0179-8. Epub 2020 Mar 16. Nat Metab. 2020. PMID: 32694775
-
Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC.Nat Commun. 2012 May 29;3:865. doi: 10.1038/ncomms1859. Nat Commun. 2012. PMID: 22643892
-
Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation.Elife. 2016 Jun 10;5:e13374. doi: 10.7554/eLife.13374. Elife. 2016. PMID: 27282387 Free PMC article.
References
-
- Adhikary, S., and M. Eilers. 2005. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 6: 635-645. - PubMed
-
- Ashrafian, H. 2006. Cancer's sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet 367: 618-621. - PubMed
-
- Bonnet, S., S. L. Archer, J. Allalunis-Turner, A. Haromy, C. Beaulieu, R. Thompson, C. T. Lee, G. D. Lopaschuk, L. Puttagunta, G. Harry, K. Hashimoto, C. J. Porter, M. A. Andrade, B. Thebaud, and E. D. Michelakis. 2007. A mitochondria-k(+) channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11: 37-51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
