The Tom1L1-clathrin heavy chain complex regulates membrane partitioning of the tyrosine kinase Src required for mitogenic and transforming activities
- PMID: 17785434
- PMCID: PMC2169060
- DOI: 10.1128/MCB.00543-07
The Tom1L1-clathrin heavy chain complex regulates membrane partitioning of the tyrosine kinase Src required for mitogenic and transforming activities
Abstract
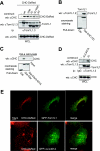
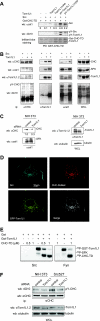
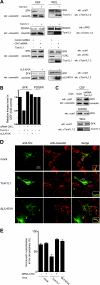
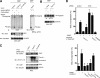
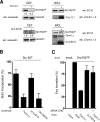
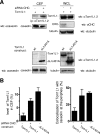
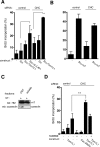
Compartmentalization of Src tyrosine kinases (SFK) plays an important role in signal transduction induced by a number of extracellular stimuli. For example, Src mitogenic signaling induced by platelet-derived growth factor (PDGF) is initiated in cholesterol-enriched microdomain caveolae. How this Src subcellular localization is regulated is largely unknown. Here we show that the Tom1L1-clathrin heavy chain (CHC) complex negatively regulates the level of SFK in caveolae needed for the induction of DNA synthesis. Tom1L1 is both an interactor and a substrate of SFK. Intriguingly, it stimulates Src activity without promoting mitogenic signaling. We found that, upon association with CHC, Tom1L1 reduced the level of SFK in caveolae, thereby preventing its association with the PDGF receptor, which is required for the induction of mitogenesis. Similarly, the Tom1L1-CHC complex reduced also the level of oncogenic Src in cholesterol-enriched microdomains, thus affecting both its capacity to induce DNA synthesis and cell transformation. Conversely, Tom1L1, when not associated with CHC, accumulated in caveolae and promoted Src-driven DNA synthesis. We concluded that the Tom1L1-CHC complex defines a novel mechanism involved in negative regulation of mitogenic and transforming signals, by modulating SFK partitioning at the plasma membrane.
Figures

References
-
- Boggon, T. J., and M. J. Eck. 2004. Structure and regulation of Src family kinases. Oncogene 23: 7918-7927. - PubMed
-
- Bonifacino, J. S. 2004. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 5: 23-32. - PubMed
-
- Boureux, A., O. Furstoss, V. Simon, and S. Roche. 2005. c-Abl tyrosine kinase regulates a Rac/JNK and a Rac/Nox pathway for DNA synthesis and c-myc expression induced by growth factors. J. Cell Sci. 118: 3717-3726. - PubMed
-
- Bromann, P. A., H. Korkaya, and S. A. Courtneidge. 2004. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23: 7957-7968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
