Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse
- PMID: 17785506
- PMCID: PMC2118451
- DOI: 10.1084/jem.20061893
Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse
Abstract
An essential function of the immunological synapse (IS) is directed secretion. NK cells are especially adept at this activity, as they direct lytic granules to the synapse for secretion, which enables cytotoxicity and facilitates host defense. This initially requires rearrangement of the actin cytoskeleton and, subsequently, microtubule-dependent trafficking of the lytic granules. As these two steps are sequential, specific linkages between them are likely to serve as critical regulators of cytotoxicity. We studied Cdc42-interacting protein-4 (CIP4), which constitutively interacts with tubulin and microtubules but focuses to the microtubule organizing center (MTOC) after NK cell activation, when it is able to associate with Wiskott-Aldrich syndrome protein (WASp) and the actin filament-rich IS. WASp deficiency, overexpression of CIP4, or parts of CIP4 interfere with this union and block normal CIP4 localization, MTOC polarization to the IS, and cytotoxicity. Reduction of endogenous CIP4 expression using small interfering RNA similarly inhibits MTOC polarization and cytotoxic activity but does not impair actin filament accumulation at the IS, or Cdc42 activation. Thus, CIP4 is an important cytoskeletal adaptor that functions after filamentous actin accumulation and Cdc42 activation to enable MTOC polarization and NK cell cytotoxicity.
Figures
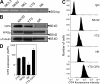
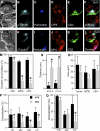
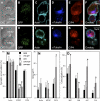
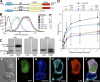




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

