HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates
- PMID: 17785525
- PMCID: PMC1950856
- DOI: 10.1101/gad.436407
HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates
Abstract
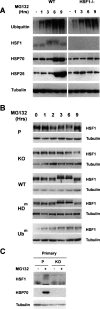
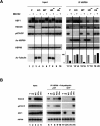
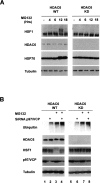
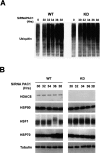
A cellular defense mechanism counteracts the deleterious effects of misfolded protein accumulation by eliciting a stress response. The cytoplasmic deacetylase HDAC6 (histone deacetylase 6) was previously shown to be a key element in this response by coordinating the clearance of protein aggregates through aggresome formation and their autophagic degradation. Here, for the first time, we demonstrate that HDAC6 is involved in another crucial cell response to the accumulation of ubiquitinated protein aggregates, and unravel its molecular basis. Indeed, our data show that HDAC6 senses ubiquitinated cellular aggregates and consequently induces the expression of major cellular chaperones by triggering the dissociation of a repressive HDAC6/HSF1 (heat-shock factor 1)/HSP90 (heat-shock protein 90) complex and a subsequent HSF1 activation. HDAC6 therefore appears as a master regulator of the cell protective response to cytotoxic protein aggregate formation.
Figures

References
-
- Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Kumaraswamy S., Boyapalle S., Atadja P., Boyapalle S., Atadja P., Atadja P., et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280:26729–26734. - PubMed
-
- Bennett E.J., Bence N.F., Jayakumar R., Kopito R.R., Bence N.F., Jayakumar R., Kopito R.R., Jayakumar R., Kopito R.R., Kopito R.R. Global impairment of the ubiquitin–proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell. 2005;17:351–365. - PubMed
-
- Bertos N.R., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Gilquin B., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Chan G.K., Yen T.J., Khochbin S., Yang X.J., Yen T.J., Khochbin S., Yang X.J., Khochbin S., Yang X.J., Yang X.J. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J. Biol. Chem. 2004;279:48246–48254. - PubMed
-
- Boyault C., Gilquin B., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Gilquin B., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Zhang Y., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Rybin V., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Garman E., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Meyer-Klaucke W., Matthias P., Muller C.W., Khochbin S., Matthias P., Muller C.W., Khochbin S., Muller C.W., Khochbin S., Khochbin S. HDAC6–p97/VCP controlled polyubiquitin chain turnover. EMBO J. 2006;25:3357–3366. - PMC - PubMed
-
- Christians E., Michel E., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Michel E., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Adenot P., Mezger V., Rallu M., Morange M., Renard J.P., Mezger V., Rallu M., Morange M., Renard J.P., Rallu M., Morange M., Renard J.P., Morange M., Renard J.P., Renard J.P. Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of the HSP70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell. Biol. 1997;17:778–788. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
