Postnatal ontogeny of skeletal muscle protein synthesis in pigs
- PMID: 17785597
- PMCID: PMC2640319
- DOI: 10.2527/jas.2007-0419
Postnatal ontogeny of skeletal muscle protein synthesis in pigs
Abstract
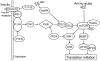
The neonatal period is characterized by rapid growth and elevated rates of synthesis and accretion of skeletal muscle proteins. The fractional rate of muscle protein synthesis is very high at birth and declines rapidly with age. The elevated capacity for muscle protein synthesis in the neonatal pig is driven by the high ribosome content and, together with an increased efficiency of the translation process, promotes accelerated protein synthesis rates. Feeding profoundly stimulates muscle protein synthesis in neonatal pigs and the response decreases with age. The feeding-induced stimulation of muscle protein synthesis is modulated by an enhanced sensitivity to the postprandial increase in insulin and amino acids. The developmental decline in the response to insulin and amino acids parallels a marked decrease in the feeding-induced activation of translation initiation factors that regulate the binding of mRNA to the 40S ribosomal complex. The abundance and activation of many known positive regulators of the nutrient- and insulin-signaling pathways that are involved in translation initiation are high, whereas those of many negative regulators are low in skeletal muscle of younger pigs. Thus, the activation and(or) abundance of the positive regulators, such as the insulin receptor, insulin receptor-substrate-1, phosphoinositide-3 kinase, phosphoinositide-dependent kinase-1, protein kinase B, mammalian target of rapamycin, raptor, ribosomal protein S6 kinase-1, eukaryotic initiation factor (eIF) 4E-binding protein 1, and eIF4E associated with eIF4G, are greater in 7-d-old pigs than in 26-d-old pigs. The activation of negative regulators, including protein tyrosine phosphatase-1B, phosphatase and tensin homologue deleted on chromosome 10, protein phosphatase 2A, and tuberous sclerosis complex 1/2, are lower in 7-d-old pigs than in 26-d-old pigs. Thus, the developmental decline in the stimulation of skeletal muscle protein synthesis by insulin and amino acids is due in part to the developmentally related decrease in the activation of the signaling pathways that lead to translation initiation.
Figures
Similar articles
-
Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs.Am J Physiol Endocrinol Metab. 2006 Oct;291(4):E849-59. doi: 10.1152/ajpendo.00069.2006. Epub 2006 Jun 6. Am J Physiol Endocrinol Metab. 2006. PMID: 16757550
-
Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated.Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1597-605. doi: 10.1152/ajpendo.00307.2007. Epub 2007 Sep 18. Am J Physiol Endocrinol Metab. 2007. PMID: 17878222 Free PMC article.
-
Stimulation of muscle protein synthesis by somatotropin in pigs is independent of the somatotropin-induced increase in circulating insulin.Am J Physiol Endocrinol Metab. 2008 Jul;295(1):E187-94. doi: 10.1152/ajpendo.90253.2008. Epub 2008 May 6. Am J Physiol Endocrinol Metab. 2008. PMID: 18460595 Free PMC article.
-
Triennial Growth Symposium: leucine acts as a nutrient signal to stimulate protein synthesis in neonatal pigs.J Anim Sci. 2011 Jul;89(7):2004-16. doi: 10.2527/jas.2010-3400. Epub 2010 Oct 8. J Anim Sci. 2011. PMID: 20935141 Free PMC article. Review.
-
Regulation of Muscle Growth in Early Postnatal Life in a Swine Model.Annu Rev Anim Biosci. 2019 Feb 15;7:309-335. doi: 10.1146/annurev-animal-020518-115130. Epub 2018 Nov 2. Annu Rev Anim Biosci. 2019. PMID: 30388025 Free PMC article. Review.
Cited by
-
The influence of dietary leucine above recommendations and fixed ratios to isoleucine and valine on muscle protein synthesis and degradation pathways in broilers.Poult Sci. 2019 Dec 1;98(12):6772-6786. doi: 10.3382/ps/pez396. Poult Sci. 2019. PMID: 31250025 Free PMC article.
-
Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs.Am J Physiol Regul Integr Comp Physiol. 2012 Mar 15;302(6):R682-90. doi: 10.1152/ajpregu.00259.2011. Epub 2012 Jan 25. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22277935 Free PMC article.
-
Shotgun proteomics of homogenate milk reveals dynamic changes in protein abundances between colostrum, transitional, and mature milk of swine.J Anim Sci. 2021 Sep 1;99(9):skab240. doi: 10.1093/jas/skab240. J Anim Sci. 2021. PMID: 34383053 Free PMC article.
-
Neonatal high protein intake enhances neonatal growth without significant adverse renal effects in spontaneous IUGR piglets.Physiol Rep. 2017 May;5(10):e13296. doi: 10.14814/phy2.13296. Physiol Rep. 2017. PMID: 28554968 Free PMC article.
-
Transcriptomic profile of leg muscle during early growth in chicken.PLoS One. 2017 Mar 14;12(3):e0173824. doi: 10.1371/journal.pone.0173824. eCollection 2017. PLoS One. 2017. PMID: 28291821 Free PMC article.
References
-
- Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: A protein kinase switching between life and death. Pharmacol Res. 2004;50:545–549. - PubMed
-
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. - PubMed
-
- Baillie AG, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. Am J Physiol. 1992;262:E1–E5. - PubMed
-
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest. 1990;20:41–50. - PubMed
-
- Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

