Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages
- PMID: 17786242
- PMCID: PMC1957542
- DOI: 10.1172/JCI32282
Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages
Abstract
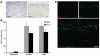
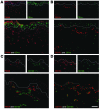
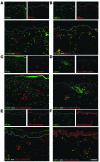
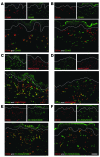
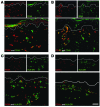
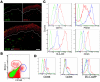
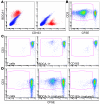
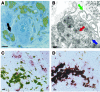
We used a panel of monoclonal antibodies to characterize DCs in the dermis of normal human skin. Staining for the CD11c integrin, which is abundant on many kinds of DCs, revealed cells in the upper dermis. These cells were positive for blood DC antigen-1 (BDCA-1; also known as CD1c), HLA-DR, and CD45, markers that are also expressed by circulating myeloid DCs. A small subset of CD11c+ dermal cells expressed DEC-205/CD205 and DC-lysosomal-associated membrane glycoprotein/CD208 (DC-LAMP/CD208), suggesting some differentiation or maturation. When BDCA-1+ cells were selected from collagenase digests of normal dermis, they proved to be strong stimulators for T cells in a mixed leukocyte reaction. A second major population of cells located throughout the dermis was positive for factor XIIIA (FXIIIA), but lacked CD11c and BDCA-1. They expressed the macrophage scavenger receptor CD163 and stained weakly for HLA-DR and CD45. Isolated CD163+ dermal cells were inactive in stimulating T cell proliferation, but in biopsies of tattoos, these cells were selectively laden with granular pigments. Plasmacytoid DCs were also present in the dermis, marked by CD123 and BDCA-2. In summary, the normal dermis contains typical immunostimulatory myeloid DCs identified by CD11c and BDCA-1, as well as an additional population of poorly stimulatory macrophages marked by CD163 and FXIIIA.
Figures

Comment in
-
Deepening our understanding of immune sentinels in the skin.J Clin Invest. 2007 Sep;117(9):2382-5. doi: 10.1172/JCI33349. J Clin Invest. 2007. PMID: 17786233 Free PMC article.
References
-
- Larregina A.T., Falo L.D. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J. Invest. Dermatol. 2005;124:1–12. - PubMed
-
- Valladeau J., Saeland S. Cutaneous dendritic cells. Semin. Immunol. 2005;17:273–283. - PubMed
-
- Cerio R., Griffiths C.E., Cooper K.D., Nickoloff B.J., Headington J.T. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br. J. Dermatol. 1989;121:421–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

