Aberrant activation of integrin alpha4beta7 suppresses lymphocyte migration to the gut
- PMID: 17786243
- PMCID: PMC1952632
- DOI: 10.1172/JCI31570
Aberrant activation of integrin alpha4beta7 suppresses lymphocyte migration to the gut
Abstract
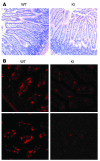
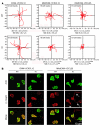
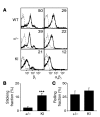
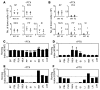
Integrin adhesion molecules mediate lymphocyte migration and homing to normal and inflamed tissues. While the ligand-binding activity of integrins is known to be modulated by conformational changes, little is known about how the appropriate balance of integrin adhesiveness is maintained in order to optimize the migratory capacity of lymphocytes in vivo. In this study we examined the regulation of the gut homing receptor alpha4beta7 integrin by manipulating at the germline level an integrin regulatory domain known as adjacent to metal ion-dependent adhesion site (ADMIDAS). ADMIDAS normally serves to raise the activation threshold of alpha4beta7, thereby stabilizing it in the default nonadhesive state. Lymphocytes from knockin beta7 (D146A) mice, which harbor a disrupted ADMIDAS, not only expressed an alpha4beta7 integrin that persistently adhered to mucosal addressin cell adhesion molecule-1 (MAdCAM-1), but also exhibited perturbed cell migration along MAdCAM-1 substrates resulting from improper de-adhesion of the lymphocyte trailing edge. In vivo, aberrantly activated alpha4beta7 enhanced adhesion to Peyer's patch venules, but suppressed lymphocyte homing to the gut, diminishing the capacity of T cells to induce colitis. Our results underscore the importance of a proper balance in the adhesion and de-adhesion of the alpha4beta7 integrin, both for lymphocyte trafficking to the gut and for colitis progression.
Figures




Comment in
-
Rheostat regulation of integrin-mediated leukocyte adhesion.J Clin Invest. 2007 Sep;117(9):2391-5. doi: 10.1172/JCI33376. J Clin Invest. 2007. PMID: 17786236 Free PMC article.
References
-
- Hynes R.O. Integrins: bi-directional, allosteric, signalling machines. Cell. 2002;110:673–687. - PubMed
-
- Carman C.V., Springer T.A. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr. Opin. Cell Biol. 2003;15:547–556. - PubMed
-
- Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 2005;5:546–559. - PubMed
-
- Lu C., Springer T.A. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1 (LFA-1). J. Immunol. 1997;159:268–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

