Recovery from diabetes in mice by beta cell regeneration
- PMID: 17786244
- PMCID: PMC1957545
- DOI: 10.1172/JCI32959
Recovery from diabetes in mice by beta cell regeneration
Abstract
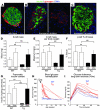
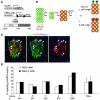
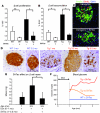
The mechanisms that regulate pancreatic beta cell mass are poorly understood. While autoimmune and pharmacological destruction of insulin-producing beta cells is often irreversible, adult beta cell mass does fluctuate in response to physiological cues including pregnancy and insulin resistance. This plasticity points to the possibility of harnessing the regenerative capacity of the beta cell to treat diabetes. We developed a transgenic mouse model to study the dynamics of beta cell regeneration from a diabetic state. Following doxycycline administration, transgenic mice expressed diphtheria toxin in beta cells, resulting in apoptosis of 70%-80% of beta cells, destruction of islet architecture, and diabetes. Withdrawal of doxycycline resulted in a spontaneous normalization of blood glucose levels and islet architecture and a significant regeneration of beta cell mass with no apparent toxicity of transient hyperglycemia. Lineage tracing analysis indicated that enhanced proliferation of surviving beta cells played the major role in regeneration. Surprisingly, treatment with Sirolimus and Tacrolimus, immunosuppressants used in the Edmonton protocol for human islet transplantation, inhibited beta cell regeneration and prevented the normalization of glucose homeostasis. These results suggest that regenerative therapy for type 1 diabetes may be achieved if autoimmunity is halted using regeneration-compatible drugs.
Figures


Comment in
-
Beta cell transplantation and immunosuppression: can't live with it, can't live without it.J Clin Invest. 2007 Sep;117(9):2380-2. doi: 10.1172/JCI33375. J Clin Invest. 2007. PMID: 17786232 Free PMC article.
References
-
- Rood P.P., et al. Facilitating physiologic self-regeneration: a step beyond islet cell replacement. Pharm. Res. 2006;23:227–242. - PubMed
-
- Parsons J.A., Brelje T.C., Sorenson R.L. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. - PubMed
-
- Parsons J.A., Bartke A., Sorenson R.L. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. - PubMed
-
- Maclean N., Ogilvie R.F. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4:367–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

